Perturbations in mitochondrial metabolism associated with defective cardiolipin biosynthesis: An in-organello real-time NMR study
- PMID: 39236875
- PMCID: PMC11470594
- DOI: 10.1016/j.jbc.2024.107746
Perturbations in mitochondrial metabolism associated with defective cardiolipin biosynthesis: An in-organello real-time NMR study
Abstract
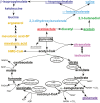
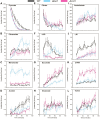
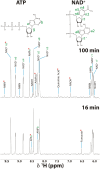
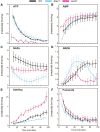
Mitochondria are central to cellular metabolism; hence, their dysfunction contributes to a wide array of human diseases. Cardiolipin, the signature phospholipid of the mitochondrion, affects proper cristae morphology, bioenergetic functions, and metabolic reactions carried out in mitochondrial membranes. To match tissue-specific metabolic demands, cardiolipin typically undergoes an acyl tail remodeling process with the final step carried out by the phospholipid-lysophospholipid transacylase tafazzin. Mutations in tafazzin are the primary cause of Barth syndrome. Here, we investigated how defects in cardiolipin biosynthesis and remodeling impacts metabolic flux through the TCA cycle and associated yeast pathways. Nuclear magnetic resonance was used to monitor in real-time the metabolic fate of 13C3-pyruvate in isolated mitochondria from three isogenic yeast strains. We compared mitochondria from a WT strain to mitochondria from a Δtaz1 strain that lacks tafazzin and contains lower amounts of unremodeled cardiolipin and mitochondria from a Δcrd1 strain that lacks cardiolipin synthase and cannot synthesize cardiolipin. We found that the 13C-label from the pyruvate substrate was distributed through twelve metabolites. Several of the metabolites were specific to yeast pathways including branched chain amino acids and fusel alcohol synthesis. While most metabolites showed similar kinetics among the different strains, mevalonate concentrations were significantly increased in Δtaz1 mitochondria. Additionally, the kinetic profiles of α-ketoglutarate, as well as NAD+ and NADH measured in separate experiments, displayed significantly lower concentrations for Δtaz1 and Δcrd1 mitochondria at most time points. Taken together, the results show how cardiolipin remodeling influences pyruvate metabolism, tricarboxylic acid cycle flux, and the levels of mitochondrial nucleotides.
Keywords: 3-methylglutaconic acid (3MGA); Barth syndrome (BTHS); Krebs cycle; adenosine triphosphate (ATP); metabolic disease; mitochondrial respiration; nuclear magnetic resonance (NMR); tricarboxylic acid (TCA) cycle.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest The author declares that they have no conflicts of interest with the contents of this article.
Figures



Update of
-
Perturbations in mitochondrial metabolism associated with defective cardiolipin biosynthesis: An in-organello real-time NMR study.bioRxiv [Preprint]. 2024 Jun 22:2024.06.18.599628. doi: 10.1101/2024.06.18.599628. bioRxiv. 2024. Update in: J Biol Chem. 2024 Oct;300(10):107746. doi: 10.1016/j.jbc.2024.107746. PMID: 38948727 Free PMC article. Updated. Preprint.
References
-
- Aversa R., Petrescu R.V.V., Apicella A., Petrescu F.I.T. Mitochondria are naturally micro robots - a review. Am. J. Eng. Appl. Sci. 2016;9:991–1002.
-
- Giacomello M., Pyakurel A., Glytsou C., Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020;21:204–224. - PubMed
-
- Murphy M.P., O’Neill L.A.J. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell. 2018;174:780–784. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

