Protocol for Health Risk Information Technology-Assisted Genetic Evaluation (HeRITAGE): a randomised controlled trial of digital genetic cancer risk assessment in a diverse underserved gynaecology clinic
- PMID: 39237276
- PMCID: PMC11381689
- DOI: 10.1136/bmjopen-2023-082658
Protocol for Health Risk Information Technology-Assisted Genetic Evaluation (HeRITAGE): a randomised controlled trial of digital genetic cancer risk assessment in a diverse underserved gynaecology clinic
Abstract
Introduction: In the USA, up to 95% of individuals harbouring cancer-predisposing germline pathogenic variants have not been identified despite recommendations for screening at the primary care level.
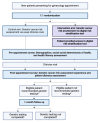
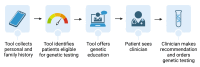
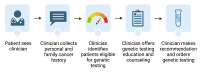
Methods and analysis: Our primary objective is to use a two-arm, single-institution randomised controlled trial to compare the proportion of eligible patients that are recommended genetic testing for hereditary cancer syndromes using a digital tool versus clinician interview for genetic cancer risk assessment in an urban academic gynaecology clinic. New gynaecology patients will be consented and randomised 1:1 to either the intervention arm, in which a digital tool is used for genetic cancer risk assessment, or usual care, in which the clinician performs genetic cancer risk assessment. Individuals will be considered eligible for hereditary cancer syndrome genetic testing if criteria set forth by the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology are met. Eligible patients are 18 years or older, speak and read English, have not yet undergone hereditary cancer genetic testing and have access to a smartphone. The study aims to enrol 50 patients in each arm to allow for 80% power with two-tailed alpha of 5% to detect a 20% difference in proportion of eligible patients recommended for genetic testing. The primary outcome is the proportion of eligible individuals recommended genetic testing in the digital tool arm versus usual care arm, analysed using the χ2 or Fisher's exact test as appropriate for sample size. The secondary outcome is completion of genetic testing, as well as exploration of patient factors, particularly social determinants of health, which may affect the receipt, utilisation and experience with genetic services.
Ethics and dissemination: This study has been approved by the Weill Cornell Institutional Review Board (Protocol No. 21-11024123). Participants will be informed of the benefits and risks of participation prior to consent. Dissemination of data will be deidentified and conducted through academic conferences and journals. Patients identified to be eligible for genetic testing who did not receive counselling from their providers will be contacted; participants will not receive direct notification of trial results.
Registration details: This trial is registered at clinicaltrials.gov (NCT05562778) in September 2022.
Protocol version: This is protocol version 1, as of 22 May 2024.
Countries of recruitment and recruitment status: USA, currently recruiting.
Health conditions/problems studied: Genetic predisposition to cancers such as breast, ovarian, uterine and pancreatic. DEIDENTIFIED INDIVIDUAL CLINICAL TRIAL PARTICIPANT-LEVEL DATA IDP SHARING STATEMENT: IDP will not be shared.
Trial registration number: NCT05562778.
Keywords: Cancer genetics; GENETICS; GYNAECOLOGY; Gynaecological oncology.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
