Long-term safety of sapropterin in paediatric and adult individuals with phenylalanine hydroxylase deficiency: Final results of the Kuvan® Adult Maternal Paediatric European Registry multinational observational study
- PMID: 39237321
- PMCID: PMC11667744
- DOI: 10.1002/jimd.12796
Long-term safety of sapropterin in paediatric and adult individuals with phenylalanine hydroxylase deficiency: Final results of the Kuvan® Adult Maternal Paediatric European Registry multinational observational study
Abstract
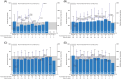
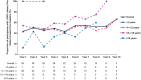
Phenylketonuria is a rare inherited disorder that disrupts the metabolism of phenylalanine (Phe) to tyrosine by phenylalanine hydroxylase (PAH). Sapropterin dihydrochloride (Kuvan®) is approved for use in Europe to reduce blood Phe levels and improve Phe tolerance in sapropterin-responsive individuals. KAMPER (NCT01016392) is an observational, multinational registry assessing long-term safety and efficacy of sapropterin. Five hundred and seventy-six participants with PAH deficiency were enrolled from nine European countries (69 sites; December 2009-May 2016). Participants were aged <4 years (n = 11), 4 to <12 years (n = 329), 12 to <18 years (n = 141), and ≥18 years (n = 95) at enrolment. Overall, 401 (69.6%) participants experienced a total of 1960 adverse events; 61 events in 42 participants were serious, and two were considered sapropterin-related by the investigator. Mean (standard deviation) actual dietary Phe intake increased from baseline across all age groups: 957 (799) mg/day to a maximum of 1959 (1121) mg/day over a total study period of 11 years. Most participants exhibited an increase in Phe tolerance while blood Phe levels remained in the target range for their age (120-360 μmol/L for <12 years; 120-600 μmol/L for ≥12 years). Most participants exhibited normal growth for height, weight, and body mass index. No additional safety concerns were identified. As an observational study, limitations include variability in routine care practices and inconsistent availability of data. Long-term sapropterin use demonstrates a favourable safety profile in real-world settings and increases Phe tolerance in participants with PAH deficiency while maintaining blood Phe levels in the target ranges.
Keywords: BH4; KAMPER; hyperphenylalaninaemia; phenylalanine hydroxylase deficiency; phenylketonuria; sapropterin dihydrochloride.
© 2024 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
François Feillet is a member of different scientific advisory boards and has received honoraria from Alexion, BioMarin, Danone, Genzyme, Immedica, Merck‐Serono, Recordati, Shire, Sobi, and Vitaflo. Jean‐Baptiste Arnoux has received honoraria for speaking engagements from Pierre Fabre Pharma and Sanofi Genzyme; fees for scientific advisory board meetings from Alexion, BioMarin, Immedica Pharma, and Zealand Pharma; fees for expert testimony from Sobi and Zealand Pharma; and travel support from Immedica Pharma. Brigitte Chabrol has participated as a clinical trial investigator for BioMarin. Ece Kucuksayrac is an employee and stockholder of BioMarin. Florian B. Lagler has received grants/contracts from Kindness for Kids Foundation, MPS Austria, Sanofi and Takeda; consulting fees, honoraria for speaking engagements, and travel support from Chiesi, Sanofi and Takeda; and consulting fees and honoraria for speaking engagements from Amicus and BioMarin. Ania C. Muntau has participated as a clinical trial investigator for BioMarin and has received consulting and speaker fees from APR, BioMarin, and PTC Therapeutics. Sabrina Paci has received consulting and speaker fees from BioMarin, Recordati Rare Diseases, Vitaflo, and Dr Schar Mevalia. Frank Rutsch has participated as a clinical trial investigator for and received consulting and speaker fees from BioMarin. Francjan J. van Spronsen declares that his institution received consulting fees for scientific advisory board meetings; grants for clinical and non‐clinical studies; honoraria for speaking engagements and clinical trial funding from BioMarin; and has received fees for advisory board meetings from Agios, alltRNA, Arla Food Int, Applied Pharma Research, Eurocept, Illumina, LogicBio, Lucane, Moderna, Nestle‐Codexis Alliance, Nutricia, Origin Biosciences, and Orphan Europe; grants from Alexion, BioMarin, ESPKU, NPKUA, NPKUV, Nutricia, PKU Research Foundation (NL), Sobi, and the Tyrosinemia Foundation; and consulting fees from Applied Pharma Research, BioMarin, Nutricia, Orphan Europe, and Pluvia Biotech. María Bueno Delgado, Alberto Burlina, and David Olsson declare that they have no conflicts of interest.
Figures



Similar articles
-
Efficacy and safety of sapropterin before and during pregnancy: Final analysis of the Kuvan® Adult Maternal Paediatric European Registry (KAMPER) maternal and Phenylketonuria Developmental Outcomes and Safety (PKUDOS) PKU-MOMs sub-registries.J Inherit Metab Dis. 2024 Jul;47(4):636-650. doi: 10.1002/jimd.12724. Epub 2024 Mar 3. J Inherit Metab Dis. 2024. PMID: 38433424
-
Long-term efficacy and safety of sapropterin in patients who initiated sapropterin at < 4 years of age with phenylketonuria: results of the 3-year extension of the SPARK open-label, multicentre, randomised phase IIIb trial.Orphanet J Rare Dis. 2021 Aug 3;16(1):341. doi: 10.1186/s13023-021-01968-1. Orphanet J Rare Dis. 2021. PMID: 34344399 Free PMC article. Clinical Trial.
-
The Kuvan(®) Adult Maternal Paediatric European Registry (KAMPER) Multinational Observational Study: Baseline and 1-Year Data in Phenylketonuria Patients Responsive to Sapropterin.JIMD Rep. 2015;23:35-43. doi: 10.1007/8904_2015_425. Epub 2015 Mar 31. JIMD Rep. 2015. PMID: 25822821 Free PMC article.
-
Sapropterin: a review of its use in the treatment of primary hyperphenylalaninaemia.Drugs. 2009;69(4):461-76. doi: 10.2165/00003495-200969040-00006. Drugs. 2009. PMID: 19323589 Review.
-
Efficacy and safety of sapropterin dihydrochloride in patients with phenylketonuria: A meta-analysis of randomized controlled trials.Br J Clin Pharmacol. 2019 May;85(5):893-899. doi: 10.1111/bcp.13886. Epub 2019 Mar 18. Br J Clin Pharmacol. 2019. PMID: 30720885 Free PMC article.
References
-
- Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376(9750):1417‐1427. - PubMed
-
- Vockley J, Andersson HC, Antshel KM, et al. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet Med. 2014;16(2):188‐200. - PubMed
-
- Bilder DA, Kobori JA, Cohen‐Pfeffer JL, Johnson EM, Jurecki ER, Grant ML. Neuropsychiatric comorbidities in adults with phenylketonuria: a retrospective cohort study. Mol Genet Metab. 2017;121(1):1‐8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

