Phase II trial of elotuzumab with pomalidomide and dexamethasone for daratumumab-refractory multiple myeloma
- PMID: 39237502
- PMCID: PMC11377826
- DOI: 10.1038/s41408-024-01134-3
Phase II trial of elotuzumab with pomalidomide and dexamethasone for daratumumab-refractory multiple myeloma
Conflict of interest statement
BRL, JE, AF, CJF, DA, DC, JB, SD and VR have no conflicts of interest to declare. RDP serves on the advisory board for Sanofi Aventis and Astra Zeneca and has received research funding from Bristol Myers Squibb Foundation and GlaxoSmithKline. SA has provided consultancy for Celgene, Amgen, Janssen and Takeda, and has received research funding from Pharmacyclics, Cellectar and Janssen. AAC-K has received research funding from Xencor Pharmacyclics, Merck, Janssen, Ascentage and Millennium.
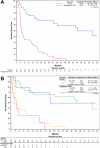
Figures
References
-
- Joseph NS, Kaufman JL, Dicamillo S, Roberts D, Gupta VA, Hofmeister CC, et al. Comparison of response and survival outcomes in standard- and high-risk newly diagnosed transplant-eligible multiple myeloma (NDMM) patients treated with lenalidomide, bortezomib and dexamethasone (RVD) versus daratumumab, lenalidomide, bortezomib and dexamethasone (D-RVD). Blood. 2023;142:647. 10.1182/blood-2023-187339 - DOI
-
- Collins SM, Bakan CE, Swartzel GD, Hofmeister CC, Efebera YA, Kwon H, et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol Immunother. 2013;62:1841–9. 10.1007/s00262-013-1493-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical