Genetic diversity within diagnostic sputum samples is mirrored in the culture of Mycobacterium tuberculosis across different settings
- PMID: 39237504
- PMCID: PMC11377819
- DOI: 10.1038/s41467-024-51266-0
Genetic diversity within diagnostic sputum samples is mirrored in the culture of Mycobacterium tuberculosis across different settings
Abstract
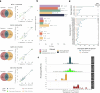
Culturing and genomic sequencing of Mycobacterium tuberculosis (MTB) from tuberculosis (TB) cases is the basis for many research and clinical applications. The alternative, culture-free sequencing from diagnostic samples, is promising but poses challenges to obtain and analyse the MTB genome. Paradoxically, culture is assumed to impose a diversity bottleneck, which, if true, would entail unexplored consequences. To unravel this paradox we generate high-quality genomes of sputum-culture pairs from two different settings after developing a workflow for sequencing from sputum and a tailored bioinformatics analysis. Careful downstream comparisons reveal sources of sputum-culture incongruences due to false positive/negative variation associated with factors like low input MTB DNA or variable genomic depths. After accounting for these factors, contrary to the bottleneck dogma, we identify a 97% variant agreement within sputum-culture pairs, with a high correlation also in the variants' frequency (0.98). The combined analysis from five different settings and more than 100 available samples shows that our results can be extrapolated to different TB epidemic scenarios, demonstrating that for the cases tested culture accurately mirrors clinical samples.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Miotto, P., Cabibbe, A. M., Borroni, E., Degano, M. & Cirillo, D. M. Role of disputed mutations in the rpob gene in interpretation of automated liquid MGIT culture results for rifampin susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol.56, e01599–17 (2018). 10.1128/JCM.01599-17 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

