BRCA genetic testing and counseling in breast cancer: how do we meet our patients' needs?
- PMID: 39237557
- PMCID: PMC11377442
- DOI: 10.1038/s41523-024-00686-8
BRCA genetic testing and counseling in breast cancer: how do we meet our patients' needs?
Abstract
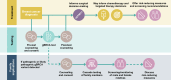
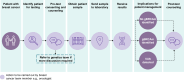
BRCA1 and BRCA2 are tumor suppressor genes that have been linked to inherited susceptibility of breast cancer. Germline BRCA1/2 pathogenic or likely pathogenic variants (gBRCAm) are clinically relevant for treatment selection in breast cancer because they confer sensitivity to poly(ADP-ribose) polymerase (PARP) inhibitors. BRCA1/2 mutation status may also impact decisions on other systemic therapies, risk-reducing measures, and choice of surgery. Consequently, demand for gBRCAm testing has increased. Several barriers to genetic testing exist, including limited access to testing facilities, trained counselors, and psychosocial support, as well as the financial burden of testing. Here, we describe current implications of gBRCAm testing for patients with breast cancer, summarize current approaches to gBRCAm testing, provide potential solutions to support wider adoption of mainstreaming testing practices, and consider future directions of testing.
© 2024. The Author(s).
Conflict of interest statement
C.J. has received consultancy fees from AstraZeneca, Daiichi Sankyo, Eli Lilly & Co., Novartis, and Roche; received support for travel or to attend meetings from Daiichi Sankyo, Pierre Fabre, and Roche; and has an unpaid role on the AGO Task Force for treatment recommendations on diagnosis and therapy in breast cancer. K.K.H. has received research funding to her institution from Cairn Surgical, Eli Lilly & Co., and Lumicell; is an Editor for
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

