IGF-I concentration determines cell fate by converting signaling dynamics as a bifurcation parameter in L6 myoblasts
- PMID: 39237579
- PMCID: PMC11377782
- DOI: 10.1038/s41598-024-71739-y
IGF-I concentration determines cell fate by converting signaling dynamics as a bifurcation parameter in L6 myoblasts
Abstract
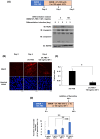
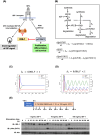
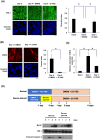
Insulin-like growth factor (IGF)-I mediates long-term activities that determine cell fate, including cell proliferation and differentiation. This study aimed to characterize the mechanisms by which IGF-I determines cell fate from the aspect of IGF-I signaling dynamics. In L6 myoblasts, myogenic differentiation proceeded under low IGF-I levels, whereas proliferation was enhanced under high levels. Mathematical and experimental analyses revealed that IGF-I signaling oscillated at low IGF-I levels but remained constant at high levels, suggesting that differences in IGF-I signaling dynamics determine cell fate. We previously reported that differential insulin receptor substrate (IRS)-1 levels generate a driving force for cell competition. Computational simulations and immunofluorescence analyses revealed that asynchronous IRS-1 protein oscillations were synchronized during myogenic processes through cell competition. Disturbances of cell competition impaired signaling synchronization and cell fusion, indicating that synchronization of IGF-I signaling oscillation is critical for myoblast cell fusion to form multinucleate myotubes.
Keywords: Bifurcation analysis; Cell competition; Insulin-like growth factor-I; L6 myoblast; Myogenesis; Signaling dynamics; Signaling oscillation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Fukushima, T. et al. Phosphatidylinositol 3-kinase (PI3K) activity bound to insulin-like growth factor-I (IGF-I) receptor, which is continuously sustained by IGF-I stimulation, is required for IGF-I-induced cell proliferation. J. Biol. Chem.287, 29713–29721. 10.1074/jbc.M112.393074 (2012). 10.1074/jbc.M112.393074 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

