New dual inducible cellular model to investigate temporal control of oncogenic cooperating genes
- PMID: 39237585
- PMCID: PMC11377716
- DOI: 10.1038/s41598-024-71227-3
New dual inducible cellular model to investigate temporal control of oncogenic cooperating genes
Abstract
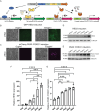
The study of cooperating genes in cancer can lead to mechanistic understanding and identifying potential therapeutic targets. To facilitate these types of studies, we developed a new dual-inducible system utilizing the tetracycline- and cumate-inducible systems driving HES3 and the PAX3::FOXO1 fusion-oncogene, respectively, as cooperating genes from fusion-positive rhabdomyosarcoma. With this model, we can independently induce expression of either HES3 or PAX3::FOXO1, as well as simultaneously induce expression of both genes. This new model will allow us to further investigate the cooperation between HES3 and PAX3::FOXO1 including the temporal requirements for genetic cooperation. Functionally, we show that dual-induction of PAX3::FOXO1 and HES3 modifies sphere formation in a HEK293T-based system. More broadly, this lentiviral dual-inducible system can be adapted for any cooperating genes (overexpression or knockdown), allowing for independent, simultaneous, or temporally controlled gene expression.
Keywords: Cooperating genes; Cumate-inducible expression; Fusion-oncogene; Inducible expression; Temporal control; Tetracycline-inducible expression.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
New Dual Inducible Cellular Model to Investigate Temporal Control of Oncogenic Cooperating Genes.bioRxiv [Preprint]. 2024 Feb 27:2024.02.23.581802. doi: 10.1101/2024.02.23.581802. bioRxiv. 2024. Update in: Sci Rep. 2024 Sep 5;14(1):20773. doi: 10.1038/s41598-024-71227-3. PMID: 38464002 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

