Small GTP-binding protein GDP dissociation stimulator influences cisplatin-induced acute kidney injury via PERK-dependent ER stress
- PMID: 39237614
- PMCID: PMC11377573
- DOI: 10.1038/s42003-024-06792-4
Small GTP-binding protein GDP dissociation stimulator influences cisplatin-induced acute kidney injury via PERK-dependent ER stress
Abstract
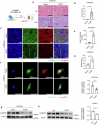
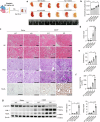
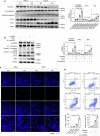
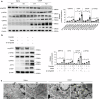
Cisplatin is a common anticancer drug, but its frequent nephrotoxicity limits its clinical use. Small GTP-binding protein GDP dissociation stimulator (smgGDS), a small GTPase chaperone protein, was considerably downregulated during cisplatin-induced acute kidney injury (CDDP-AKI), especially in renal tubular epithelial cells. SmgGDS-knockdown mice was established and found that smgGDS knockdown promoted CDDP-AKI, as demonstrated by an increase in serum creatine, blood urea nitrogen levels and the appearance of tubular patterns. RNA sequencing suggested that protein kinase RNA-like ER kinase (PERK), which bridges mitochondria-associated ER membranes, was involved in smgGDS knockdown following CDDP-AKI, and then identified that smgGDS knockdown increased phosphorylated-PERK in vivo and in vitro. Furthermore, we confirmed that smgGDS deficiency aggravated apoptosis and ER stress in vivo and in vitro. And the ER stress inhibitor 4-Phenylbutyric acid and the inhibition of PERK phosphorylation mitigated smgGDS deficiency-induced ER stress related apoptosis following cisplatin treatment, while the eIF2α phosphorylation inhibitor could not reverse the smgGDS deficiency accelerated cell death. Furthermore, the over-expression of smgGDS could reverse the ER stress and apoptosis caused by CDDP. Overall, smgGDS regulated PERK-dependent ER stress and apoptosis, thereby influencing renal damage. This study identified a target for diagnosing and treating cisplatin-induced acute kidney injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests. The corresponding author, Daxin Wang, had full access to all data in the study and had the final responsibility for the decision to submit for publication.
Figures


Similar articles
-
Effects of ginsenoside Rh2 on cisplatin-induced nephrotoxicity in renal tubular epithelial cells by inhibiting endoplasmic reticulum stress.J Biochem Mol Toxicol. 2024 Aug;38(8):e23768. doi: 10.1002/jbt.23768. J Biochem Mol Toxicol. 2024. PMID: 39015062
-
Pharmacological and genetic inhibition of fatty acid-binding protein 4 alleviated cisplatin-induced acute kidney injury.J Cell Mol Med. 2019 Sep;23(9):6260-6270. doi: 10.1111/jcmm.14512. Epub 2019 Jul 8. J Cell Mol Med. 2019. PMID: 31286669 Free PMC article.
-
Phosphorylation of eIF2α suppresses cisplatin-induced p53 activation and apoptosis by attenuating oxidative stress via ATF4-mediated HO-1 expression in human renal proximal tubular cells.Int J Mol Med. 2017 Dec;40(6):1957-1964. doi: 10.3892/ijmm.2017.3181. Epub 2017 Oct 10. Int J Mol Med. 2017. PMID: 29039478
-
Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury.Ann Med. 2018 Aug;50(5):381-390. doi: 10.1080/07853890.2018.1489142. Epub 2018 Jul 11. Ann Med. 2018. PMID: 29895209 Free PMC article. Review.
-
Targeting Endoplasmic Reticulum Stress by Natural and Chemical Compounds Ameliorates Cisplatin-Induced Nephrotoxicity: A Review.Biol Trace Elem Res. 2025 May;203(5):2687-2700. doi: 10.1007/s12011-024-04351-w. Epub 2024 Aug 30. Biol Trace Elem Res. 2025. PMID: 39212819 Review.
Cited by
-
Research progress on endoplasmic reticulum homeostasis in acute kidney injury.Front Pharmacol. 2025 Jun 20;16:1595845. doi: 10.3389/fphar.2025.1595845. eCollection 2025. Front Pharmacol. 2025. PMID: 40620676 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

