The scaffolding function of LSD1 controls DNA methylation in mouse ESCs
- PMID: 39237615
- PMCID: PMC11377572
- DOI: 10.1038/s41467-024-51966-7
The scaffolding function of LSD1 controls DNA methylation in mouse ESCs
Abstract
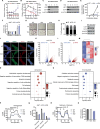
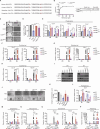
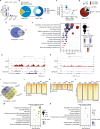
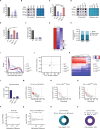
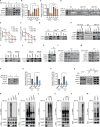
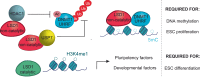
Lysine-specific histone demethylase 1 (LSD1), which demethylates mono- or di- methylated histone H3 on lysine 4 (H3K4me1/2), is essential for early embryogenesis and development. Here we show that LSD1 is dispensable for mouse embryonic stem cell (ESC) self-renewal but is required for mouse ESC growth and differentiation. Reintroduction of a catalytically-impaired LSD1 (LSD1MUT) recovers the proliferation capability of mouse ESCs, yet the enzymatic activity of LSD1 is essential to ensure proper differentiation. Indeed, increased H3K4me1 in Lsd1 knockout (KO) mouse ESCs does not lead to major changes in global gene expression programs related to stemness. However, ablation of LSD1 but not LSD1MUT results in decreased DNMT1 and UHRF1 proteins coupled to global hypomethylation. We show that both LSD1 and LSD1MUT control protein stability of UHRF1 and DNMT1 through interaction with HDAC1 and the ubiquitin-specific peptidase 7 (USP7), consequently, facilitating the deacetylation and deubiquitination of DNMT1 and UHRF1. Our studies elucidate a mechanism by which LSD1 controls DNA methylation in mouse ESCs, independently of its lysine demethylase activity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

