Phase 3 study of gilteritinib versus salvage chemotherapy in predominantly Asian patients with relapsed/refractory FLT3-mutated acute myeloid leukemia
- PMID: 39237634
- PMCID: PMC11518977
- DOI: 10.1038/s41375-024-02382-9
Phase 3 study of gilteritinib versus salvage chemotherapy in predominantly Asian patients with relapsed/refractory FLT3-mutated acute myeloid leukemia
Abstract
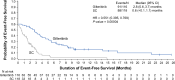
The phase 3 COMMODORE trial evaluated gilteritinib versus salvage chemotherapy (SC) in a predominantly Asian relapsed/refractory (R/R) FLT3-mutated (FLT3mut+) acute myeloid leukemia (AML) patient population. The primary endpoint was overall survival (OS); secondary endpoints included event-free survival (EFS) and complete remission (CR) rate. As of June 30, 2020 (interim analysis: 32.2 months after study initiation), 234 patients were randomized (gilteritinib, n = 116; SC, n = 118). Median OS was significantly longer with gilteritinib versus SC (9.6 vs. 5.0 months; HR 0.566 [95% CI: 0.392, 0.818]; p = 0.00211) with a median follow-up of 10.3 months. Median EFS was also significantly longer with gilteritinib (2.8 vs. 0.6 months; HR 0.551 [95% CI: 0.395, 0.769]; p = 0.00004). CR rates with gilteritinib and SC were 16.4% and 10.2%, respectively; composite CR rates were 50.0% and 20.3%, respectively. Exposure-adjusted grade ≥3 adverse event (AE) rates were lower with gilteritinib (58.38 events/patient-year [E/PY]) versus SC (168.30 E/PY). Common AEs with gilteritinib were anemia (77.9%) and thrombocytopenia (45.1%). Gilteritinib plasma concentration peaked ~4 h postdose; ~3-fold accumulation occurred with multiple dosing. The COMMODORE trial demonstrated that gilteritinib significantly improved OS and EFS in predominantly Asian patients, validating the outcomes of gilteritinib from the ADMIRAL trial in R/R FLT3mut+ AML.
© 2024. The Author(s).
Conflict of interest statement
MY, TS, TK, MT, MK, XM, and NH are all employees of Astellas. TS reports Astellas stocks. BJ, JL, LL, XD, HJ, LG, JT, LWLL, AK, and EM report consulting and honoraria fees from Astellas. JW, JH, and SB have nothing to disclose.
Figures

References
-
- Moreno I, Martin G, Bolufer P, Barragan E, Rueda E, Roman J, et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88:19–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

