Development of a New Dry Powder Aerosol Synthetic Lung Surfactant Product for Neonatal Respiratory Distress Syndrome (RDS) - Part II: In vivo Efficacy Testing in a Rabbit Surfactant Washout Model
- PMID: 39237797
- PMCID: PMC11436456
- DOI: 10.1007/s11095-024-03754-7
Development of a New Dry Powder Aerosol Synthetic Lung Surfactant Product for Neonatal Respiratory Distress Syndrome (RDS) - Part II: In vivo Efficacy Testing in a Rabbit Surfactant Washout Model
Abstract
Purpose: Surfactant therapy incorporates liquid bolus instillation via endotracheal tube catheter and a mechanical ventilator in preterm neonates with respiratory distress syndrome (RDS). Aerosolized surfactants have generated interest and conflicting data on the efficacy of phospholipid (PL) dose requirements. We developed and characterized a synthetic lung surfactant excipient enhanced growth (SLS-EEG) dry powder aerosol product. In this study, we compare the in vivo performance of the new aerosol product with standard-of-care liquid instillation.
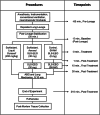
Methods: Juvenile rabbits were sedated, anesthetized, intubated, and ventilated. Endogenous surfactant was depleted via whole lung lavage. Animals received either a standard dose of liquid Curosurf (200 mg PL/kg) instilled via a tracheal catheter, SLS-EEG powder aerosol (60 mg device loaded dose; equivalent to 24 mg PL/kg), or sham control. Gas exchange, lung compliance, and indices of disease severity were recorded every 30 min for 3.5 h and macro- and microscopy images were acquired at necropsy.
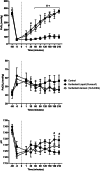
Results: While aerosol was administered at an approximately tenfold lower PL dose, both liquid-instilled and aerosol groups had similar, nearly complete recoveries of arterial oxygenation (PaO2; 96-100% recovery) and oxygenation index, and the aerosol group had superior recovery of compliance (P < 0.05). The SLS-EEG aerosol group showed less lung tissue injury, greater uniformity in lung aeration, and more homogenous surfactant distribution at the alveolar surfaces compared with liquid Curosurf.
Conclusions: The new dry powder aerosol SLS product (which includes the delivery strategy, formulation, and delivery system) has the potential to be a safe, effective, and economical alternative to the current clinical standard of liquid bolus surfactant instillation.
Keywords: infant aerosol therapy; respiratory distress syndrome; surfactant aerosol; surfactant replacement therapy; synthetic lung surfactant.
© 2024. The Author(s).
Conflict of interest statement
Virginia Commonwealth University is currently pursuing patent protection of formulations, devices, and methods described in this study. If licensed and commercialized, these may provide future financial interest to the authors.
Figures



References
-
- Ohuma EO, Moller AB, Bradley E, Chakwera S, Hussain-Alkhateeb L, Lewin A, Okwaraji YB, Mahanani WR, Johansson EW, Lavin T, Fernandez DE, Dominguez GG, de Costa A, Cresswell JA, Krasevec J, Lawn JE, Blencowe H, Requejo J, Moran AC. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. 2023;402(10409):1261–71. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

