Randomized open-label study of second-generation antipsychotics for the treatment of schizophrenia: 104-week final results of the JUMPs study assessing treatment discontinuation, remission, and social functioning
- PMID: 39237918
- PMCID: PMC11376064
- DOI: 10.1186/s12888-024-06031-4
Randomized open-label study of second-generation antipsychotics for the treatment of schizophrenia: 104-week final results of the JUMPs study assessing treatment discontinuation, remission, and social functioning
Abstract
Background: We report the final results of treatment with aripiprazole, blonanserin, and paliperidone from the Japan Useful Medication Program for Schizophrenia (JUMPs), a 104-week naturalistic study.
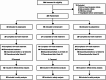
Methods: JUMPs was an open-label, three-arm, randomized, parallel-group, 104-week study. Patients aged ≥ 20 years with schizophrenia requiring antipsychotic treatment or a switch from previous therapy were enrolled. The primary endpoint was treatment discontinuation rate over 104 weeks. Secondary endpoints included remission rate, Personal and Social Performance (PSP), safety, Positive and Negative Syndrome Scale (PANSS), and quality of life (QOL; EuroQol-5 dimension).
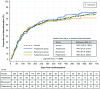
Results: In total, 251 patients received aripiprazole (n = 82), blonanserin (n = 85), or paliperidone (n = 84). Treatment discontinuation rates (aripiprazole, 80.5%; blonanserin, 81.2%; paliperidone, 71.4%) were not significantly different (p = 0.2385) among the treatment groups at 104 weeks; comparable outcomes were observed for endpoints, including remission (42.9%, 46.7%, and 45.8%), PANSS, and safety. In the overall cohort, while the improvement in the PSP total score at Week 104 was not significantly different from baseline, a significant improvement (p < 0.05) in QOL and total PANSS scores (including all subscales) was observed at Week 104 compared with baseline. Multivariable analysis identified a shorter disease duration and a higher chlorpromazine-equivalent antipsychotic dosage level (≥ 1000 mg) before switching to monotherapy as predictors of treatment discontinuation.
Conclusions: The 104-week treatment outcomes were comparable between groups; the overall trend of improvement in remission rate, safety, and QOL suggests the importance of continued treatment.
Clinical trial registration: UMIN-Clinical Trials Registry UMIN000007942 (public release date: 14/05/2012).
Keywords: 104-week effectiveness; Discontinuation rate; Monotherapy; Real-world outcomes; Remission rate; Second-generation antipsychotics.
© 2024. The Author(s).
Conflict of interest statement
Conflicts of interestJun Ishigooka reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work; payment or honoraria from Astellas Pharma Inc. (chair), Mochida Pharmaceutical Co., Ltd. (chair), Otsuka Pharmaceutical Co. Ltd. (adviser), Takeda Pharmaceutical Co., Ltd. (speaker, adviser), Novartis Pharma K.K. (adviser), and Alfresa Pharma Corporation (adviser) in the past 36 months; and support for transportation to attend meetings from Astellas Pharma Inc. and Mochida Pharmaceutical Co., Ltd. in the past 36 months. Kazuyuki Nakagome reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work; grants paid to his institution from Shionogi & Co., Ltd., Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corp., Nippon Boehringer Ingelheim Co., Ltd., and Mochida Pharmaceutical Co., Ltd. in the past 36 months; payment or honoraria from Sumitomo Pharma Co., Ltd. (speaker, chair, adviser, interview), Otsuka Pharmaceutical Co., Ltd. (speaker, chair, adviser, supervisor), Meiji Seika Pharma Co., Ltd. (speaker), Janssen Pharmaceutical K.K. (speaker, chair, supervisor panelist), Mitsubishi Tanabe Pharma Corp. (chair), Takeda Pharmaceutical Co., Ltd. (adviser), Lundbeck Japan (adviser), Viatris Inc. (chair), Eisai Co., Ltd. (chair), Nippon Boehringer Ingelheim Co., Ltd. (chair, adviser, supervisor), and Mochida Pharmaceutical Co., Ltd. (speaker) in the past 36 months; and support for transportation to attend meetings from Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corp., Takeda Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., and Mochida Pharmaceutical Co., Ltd. in the past 36 months. Tetsuro Ohmori reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work; and payment or honoraria as a chair or speaker from Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Eli Lilly Japan K.K., Takeda Pharmaceutical Co., Ltd., Eisai Co., Ltd., Yoshitomi Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., Meiji Seika Pharma Co., Ltd., Tsumura Co., Ltd., and MSD Co., Ltd. in the past 36 months. Nakao Iwata reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Viatris Inc., Janssen Pharmaceutical K.K., Meiji Seika Pharma Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd, Eli Lilly Japan K.K., and Eisai Co., Ltd. in the past 36 months. Ken Inada reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work; grants from Mochida Pharmaceutical Co., Ltd. and Sumitomo Pharma Co., Ltd. in the past 36 months; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Daiichi Sankyo, Eisai Co., Ltd., Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Lundbeck Japan, Meiji Seika Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corp., Mochida Pharmaceutical Co., Ltd., MSD Co., Ltd., Nipro Pharma Corp, Novartis, Otsuka Pharmaceutical Co., Ltd., Pfizer, Shionogi & Co., Ltd., Sumitomo Pharma Co., Ltd., Yoshitomiyakuhin Corp., and Viatris Inc. in the past 36 months. Jun-ichi Iga reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Eisai Co., Ltd., Janssen Pharmaceutical K.K., Merck, Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Kyowa Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Yoshitomiyakuhin Corp., Daiichi Sankyo, Viatris Inc., and Shionogi & Co., Ltd. in the past 36 months. Taro Kishi reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work; grants from Eisai Co., Ltd. in the past 36 months; and honoraria for speaker from Sumitomo Pharma Co., Ltd., Eisai Co., Ltd., Janssen Pharmaceutical K.K., Meiji Seika Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Viatris Inc., Mitsubishi Tanabe Pharma Corp., and MSD Co., Ltd. in the past 36 months. Kiyoshi Fujita reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co., Ltd, and Meiji Seika Pharma Co., Ltd. in the past 36 months. Hideaki Tabuse reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work; clinical trial expense for his institution from AbbVie GK, Janssen Pharmaceutical K.K., and Otsuka Pharmaceutical Co., Ltd.; payment or honoraria as conference chair from Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Viatris Inc. in the past 36 months; and transportation/accommodation fee from Janssen Pharmaceutical K.K., MSD Co., Ltd., and Takeda Pharmaceutical Co., Ltd. Hiroshi Terada reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work, and payment or honoraria for lecture/conference chair from Meiji Seika Pharma Co., Ltd., Lundbeck Japan K.K., Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Yoshitomiyakuhin Corp., Janssen Pharmaceutical K.K., and Eli Lilly Japan K.K. in the past 36 months. Haruko Terada reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work, and payment or honoraria for lecture/conference chair from Sumitomo Pharma Co., Ltd. Toshiaki Shichijo reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work; clinical trial fees paid to the institution from Sumitomo Pharma Co., Ltd. and Shionogi & Co., Ltd. in the past 36 months; and lecture honoraria from Otsuka Pharmaceutical Co., Ltd. and Sumitomo Pharma Co., Ltd. in the past 36 months. Yuichiro Tsutsumi reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work, and lecture honoraria from Janssen Pharmaceutical K.K., Sumitomo Pharma Co., Ltd., and Mitsubishi Tanabe Pharma Corp. in the past 36 months. Shotatsu Koretsune reports funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work, and honoraria for a conference chair from Takeda Pharmaceutical Co. Ltd. in the past 36 months. Yuka Kikuchi, Toshifumi Kishimoto, and Kazutaka Ohi report funding for writing support from the Waksman Foundation of Japan, Inc., since the initial planning of the work.
Figures
References
-
- Brissos S, Dias VV, Balanzá-Martinez V, Carita AI, Figueira ML. Symptomatic remission in schizophrenia patients: relationship with social functioning, quality of life, and neurocognitive performance. Schizophr Res. 2011;129(2–3):133–6. 10.1016/j.schres.2011.04.001. 10.1016/j.schres.2011.04.001 - DOI - PubMed
-
- Lieberman JA, Stroup TS, McEvoy JP, Clinical Antipsychotic Trials of Intervention Effectiveness, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23. 10.1056/NEJMoa051688. - PubMed
-
- Kahn RS, Fleischhacker WW, Boter H, EUFEST Study Group, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085–97. 10.1016/S0140-6736(08)60486-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous