Neuroprotective effects of intranasal extracellular vesicles from human platelet concentrates supernatants in traumatic brain injury and Parkinson's disease models
- PMID: 39237980
- PMCID: PMC11375990
- DOI: 10.1186/s12929-024-01072-z
Neuroprotective effects of intranasal extracellular vesicles from human platelet concentrates supernatants in traumatic brain injury and Parkinson's disease models
Abstract
Background: The burgeoning field of regenerative medicine has significantly advanced with recent findings on biotherapies using human platelet lysates (HPLs), derived from clinical-grade platelet concentrates (PCs), for treating brain disorders. These developments have opened new translational research avenues to explore the neuroprotective effects of platelet-extracellular vesicles (PEVs). Their potential in managing neurodegenerative conditions like traumatic brain injury (TBI) and Parkinson's disease (PD) warrants further exploration. We aimed here to characterize the composition of a PEV preparation isolated from platelet concentrate (PC) supernatant, and determine its neuroprotective potential and neurorestorative effects in cellular and animal models of TBI and PD.
Methods: We isolated PEVs from the supernatant of clinical-grade PC collected from healthy blood donors utilizing high-speed centrifugation. PEVs were characterized by biophysical, biochemical, microscopic, and LC-MS/MS proteomics methods to unveil biological functions. Their functionality was assessed in vitro using SH-SY5Y neuronal cells, LUHMES dopaminergic neurons, and BV-2 microglial cells, and in vivo by intranasal administration in a controlled cortical impact (CCI)-TBI model using 8-weeks-old male C57/BL6 mice, and in a PD model induced by MPTP in 5-month-old male C57/BL6 mice.
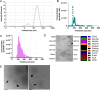
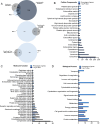
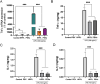
Results: PEVs varied in size from 50 to 350 nm, predominantly around 200 nm, with concentrations ranging between 1010 and 1011/mL. They expressed specific platelet membrane markers, exhibited a lipid bilayer by cryo-electron microscopy and, importantly, showed low expression of pro-coagulant phosphatidylserine. LC-MS/MS indicated a rich composition of trophic factors, including neurotrophins, anti-inflammatory agents, neurotransmitters, and antioxidants, unveiling their multifaceted biological functions. PEVs aided in the restoration of neuronal functions in SH-SY5Y cells and demonstrated remarkable neuroprotective capabilities against erastin-induced ferroptosis in dopaminergic neurons. In microglial cells, they promoted anti-inflammatory responses, particularly under inflammatory conditions. In vivo, intranasally delivered PEVs showed strong anti-inflammatory effects in a TBI mouse model and conserved tyrosine hydroxylase expression of dopaminergic neurons of the substantia nigra in a PD model, leading to improved motor function.
Conclusions: The potential of PEV-based therapies in neuroprotection opens new therapeutic avenues for neurodegenerative disorders. The study advocates for clinical trials to establish the efficacy of PEV-based biotherapies in neuroregenerative medicine.
Keywords: Blood; Central Nervous System; Exosomes; Neurological disorders; Neuroprotection.
© 2024. The Author(s).
Conflict of interest statement
The authors of this manuscript have no conflicts of interest.
Figures
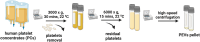



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

