Regional desynchronization of microglial activity is associated with cognitive decline in Alzheimer's disease
- PMID: 39238030
- PMCID: PMC11375924
- DOI: 10.1186/s13024-024-00752-6
Regional desynchronization of microglial activity is associated with cognitive decline in Alzheimer's disease
Abstract
Background: Microglial activation is one hallmark of Alzheimer disease (AD) neuropathology but the impact of the regional interplay of microglia cells in the brain is poorly understood. We hypothesized that microglial activation is regionally synchronized in the healthy brain but experiences regional desynchronization with ongoing neurodegenerative disease. We addressed the existence of a microglia connectome and investigated microglial desynchronization as an AD biomarker.
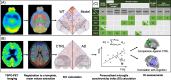
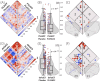
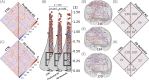
Methods: To validate the concept, we performed microglia depletion in mice to test whether interregional correlation coefficients (ICCs) of 18 kDa translocator protein (TSPO)-PET change when microglia are cleared. Next, we evaluated the influence of dysfunctional microglia and AD pathophysiology on TSPO-PET ICCs in the mouse brain, followed by translation to a human AD-continuum dataset. We correlated a personalized microglia desynchronization index with cognitive performance. Finally, we performed single-cell radiotracing (scRadiotracing) in mice to ensure the microglial source of the measured desynchronization.
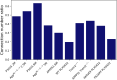
Results: Microglia-depleted mice showed a strong ICC reduction in all brain compartments, indicating microglia-specific desynchronization. AD mouse models demonstrated significant reductions of microglial synchronicity, associated with increasing variability of cellular radiotracer uptake in pathologically altered brain regions. Humans within the AD-continuum indicated a stage-depended reduction of microglia synchronicity associated with cognitive decline. scRadiotracing in mice showed that the increased TSPO signal was attributed to microglia.
Conclusion: Using TSPO-PET imaging of mice with depleted microglia and scRadiotracing in an amyloid model, we provide first evidence that a microglia connectome can be assessed in the mouse brain. Microglia synchronicity is closely associated with cognitive decline in AD and could serve as an independent personalized biomarker for disease progression.
Keywords: Alzheimer’s disease; Brain connectivity; Dementia; Microglia; Microglia desynchronization; Microglia synchronicity; Neuroinflammation; PET; TSPO.
© 2024. The Author(s).
Conflict of interest statement
NLA and MBr are members of the Neuroimaging Committee of the EANM. RR has received consultancy honoraria from SAGE and GABA Therapeutics. NF has received consultancy honoraria from MSD and speaker honoraria from LMI. MB received speaker honoraria from Roche, GE healthcare and Life Molecular Imaging and is an advisor of Life Molecular Imaging. KB received speaker honoraria or travel support by Lilly Deutschland, Eisai, Roche, Biogen, Novo Nordisk. All other authors declare they have no competing interests.
Figures






References
-
- Rauchmann BS, Brendel M, Franzmeier N, Trappmann L, Zaganjori M, Ersoezlue E, et al. Microglial Activation and Connectivity in Alzheimer Disease and Aging. Ann Neurol. 2022;92(5):768–81. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

