Targeting stress induction of GRP78 by cardiac glycoside oleandrin dually suppresses cancer and COVID-19
- PMID: 39238058
- PMCID: PMC11378597
- DOI: 10.1186/s13578-024-01297-3
Targeting stress induction of GRP78 by cardiac glycoside oleandrin dually suppresses cancer and COVID-19
Abstract
Background: Despite recent therapeutic advances, combating cancer resistance remains a formidable challenge. The 78-kilodalton glucose-regulated protein (GRP78), a key stress-inducible endoplasmic reticulum (ER) chaperone, plays a crucial role in both cancer cell survival and stress adaptation. GRP78 is also upregulated during SARS-CoV-2 infection and acts as a critical host factor. Recently, we discovered cardiac glycosides (CGs) as novel suppressors of GRP78 stress induction through a high-throughput screen of clinically relevant compound libraries. This study aims to test the possibility that agents capable of blocking stress induction of GRP78 could dually suppress cancer and COVID-19.
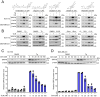
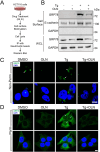
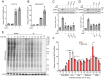
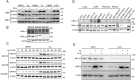
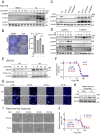
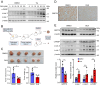
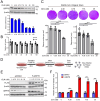
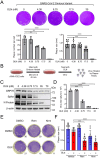
Results: Here we report that oleandrin (OLN), is the most potent among the CGs in inhibiting acute stress induction of total GRP78, which also results in reduced cell surface and nuclear forms of GRP78 in stressed cells. The inhibition of stress induction of GRP78 is at the post-transcriptional level, independent of protein degradation and autophagy and may involve translational control as OLN blocks stress-induced loading of ribosomes onto GRP78 mRNAs. Moreover, the human Na+/K+-ATPase α3 isoform is critical for OLN suppression of GRP78 stress induction. OLN, in nanomolar range, enhances apoptosis, sensitizes colorectal cancer cells to chemotherapeutic agents, and reduces the viability of patient-derived colon cancer organoids. Likewise, OLN, suppresses GRP78 expression and impedes tumor growth in an orthotopic breast cancer xenograft model. Furthermore, OLN blocks infection by SARS-CoV-2 and its variants and enhances existing anti-viral therapies. Notably, GRP78 overexpression mitigates OLN-mediated cancer cell apoptotic onset and suppression of virus release.
Conclusion: Our findings validate GRP78 as a target of OLN anti-cancer and anti-viral activities. These proof-of-principle studies support further investigation of OLN as a readily accessible compound to dually combat cancer and COVID-19.
Keywords: Anti-cancer therapy; Anti-viral therapy; Breast cancer; Cardiac glycosides; Colon cancer; ER stress; GRP78; Oleandrin; SARS-CoV-2.
© 2024. The Author(s).
Conflict of interest statement
A.S.L. is a scientific advisory board member of BiPER Therapeutics. M.Y. is the founder and president of CanTraCer Biosciences Inc. The other co-authors declare no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

