Osteopontin deletion attenuates cyst growth but exacerbates fibrosis in mice with cystic kidney disease
- PMID: 39238069
- PMCID: PMC11377176
- DOI: 10.14814/phy2.70038
Osteopontin deletion attenuates cyst growth but exacerbates fibrosis in mice with cystic kidney disease
Abstract
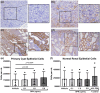
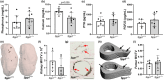
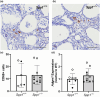
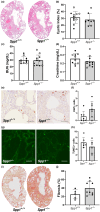
Osteopontin (OPN) is a multi-functional glycoprotein that coordinates the innate immune response, prevents nanocrystal formation in renal tubule fluid, and is a biomarker for kidney injury. OPN expression is markedly increased in cystic epithelial cells of polycystic kidney disease (PKD) kidneys; however, its role in PKD progression remains unclear. We investigated the in vitro effects of recombinant OPN on the proliferation of tubular epithelial cells from PKD and normal human kidneys and in vivo effects of OPN deletion on kidney cyst formation, fibrosis, and mineral metabolism in pcy/pcy mice, a non-orthologous model of autosomal-dominant PKD. In vitro studies revealed that OPN enhanced the proliferation of PKD cells but had no effect on normal kidney cells. Deletion of OPN in pcy/pcy mice significantly reduced kidney cyst burden; however, this was accompanied by increased fibrosis and no change in kidney function. The loss of OPN had no effect on kidney macrophage numbers, cyst epithelial cell proliferation, or apoptosis. Furthermore, there was no difference in kidney mineral deposition or mineral metabolism parameters between pcy/pcy mice with and without OPN expression. Global deletion of OPN reduced kidney cyst burden, while paradoxically exacerbating kidney fibrosis in mice with cystic kidney disease.
Keywords: Osteopontin; PKD; fibrosis; matricellular proteins; mineral metabolism.
© 2024 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
DPW has received research funding from Synkine Therapeutics. All other authors have nothing to disclose.
Figures


References
-
- Anders, H. J. , Suarez‐Alvarez, B. , Grigorescu, M. , Foresto‐Neto, O. , Steiger, S. , Desai, J. , Marschner, J. A. , Honarpisheh, M. , Shi, C. , Jordan, J. , Muller, L. , Burzlaff, N. , Bauerle, T. , & Mulay, S. R. (2018). The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis‐related chronic kidney disease independent from IL‐1‐mediated tissue injury. Kidney International, 93, 656–669. - PubMed
-
- Cowley, B. D., Jr. , Ricardo, S. D. , Nagao, S. , & Diamond, J. R. (2001). Increased renal expression of monocyte chemoattractant protein‐1 and osteopontin in ADPKD in rats. Kidney International, 60, 2087–2096. - PubMed
-
- Cui, R. , Takahashi, F. , Ohashi, R. , Gu, T. , Yoshioka, M. , Nishio, K. , Ohe, Y. , Tominaga, S. , Takagi, Y. , Sasaki, S. , Fukuchi, Y. , & Takahashi, K. (2007). Abrogation of the interaction between osteopontin and alphavbeta3 integrin reduces tumor growth of human lung cancer cells in mice. Lung Cancer, 57, 302–310. - PubMed
MeSH terms
Substances
Grants and funding
- R01DK122212/HHS | NIH | NIDDK | Division of Diabetes, Endocrinology, and Metabolic Diseases (DEM)
- R01 DK129255/DK/NIDDK NIH HHS/United States
- K01 DK119375/DK/NIDDK NIH HHS/United States
- R01DK129255/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R01 DK122212/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

