Generation of an Isogenic Hereditary Hemorrhagic Telangiectasia Model via Prime Editing in Human Induced Pluripotent Stem Cells
- PMID: 39238188
- PMCID: PMC11612218
- DOI: 10.15283/ijsc24084
Generation of an Isogenic Hereditary Hemorrhagic Telangiectasia Model via Prime Editing in Human Induced Pluripotent Stem Cells
Abstract
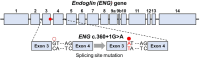
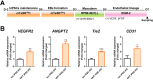
Prime editing (PE) is a recently developed genome-editing technique that enables versatile editing. Despite its flexibility and potential, applying PE in human induced pluripotent stem cells (hiPSCs) has not been extensively addressed. Genetic disease models using patient-derived hiPSCs have been used to study mechanisms and drug efficacy. However, genetic differences between patient and control cells have been attributed to the inaccuracy of the disease model, highlighting the significance of isogenic hiPSC models. Hereditary hemorrhagic telangiectasia 1 (HHT1) is a genetic disorder caused by an autosomal dominant mutation in endoglin (ENG). Although previous HHT models using mice and HUVEC have been used, these models did not sufficiently elucidate the relationship between the genotype and disease phenotype in HHT, demanding more clinically relevant models that reflect human genetics. Therefore, in this study, we used PE to propose a method for establishing an isogenic hiPSC line. Clinically reported target mutation in ENG was selected, and a strategy for PE was designed. After cloning the engineered PE guide RNA, hiPSCs were nucleofected along with PEmax and hMLH1dn plasmids. As a result, hiPSC clones with the intended mutation were obtained, which showed no changes in pluripotency or genetic integrity. Furthermore, introducing the ENG mutation increased the expression of proangiogenic markers during endothelial organoid differentiation. Consequently, our results suggest the potential of PE as a toolkit for establishing isogenic lines, enabling disease modeling based on hiPSC-derived disease-related cells or organoids. This approach is expected to stimulate mechanistic and therapeutic studies on genetic diseases.
Keywords: Endoglin; Gene editing; Genetic diseases; Hereditary hemorrhagic telangiectasia; Induced pluripotent stem cells.
Conflict of interest statement
There is no potential conflict of interest to declare.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

