Cerebellar transcranial magnetic stimulation improves motor function in Parkinson's disease
- PMID: 39238196
- PMCID: PMC11514926
- DOI: 10.1002/acn3.52183
Cerebellar transcranial magnetic stimulation improves motor function in Parkinson's disease
Abstract
Objective: To determine whether an accelerated protocol of 48 Hz cerebellar repetitive transcranial magnetic stimulation results in improved motor function in individuals with Parkinson's disease.
Methods: In this double-blind randomized sham-controlled study, 35 individuals with Parkinson's disease and stable medical treatment were randomized to either sham or verum transcranial magnetic stimulation. The stimulation was applied bilaterally and medial over the cerebellum and comprised a novel accelerated protocol encompassing two sessions per day on 5 consecutive days. Patients were assessed at baseline, on day 5 after the last stimulation and 1 month post intervention. Measurements included dynamic posturography, UPDRS III, 8-Meter walk test, and Timed Up and Go test.
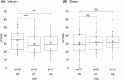
Results: The accelerated protocol was safe and feasible in an outpatient setting. Patients in the verum group showed significant improvement (p < 0.001) of motor symptoms as measured in the UPDRS III. Improvement was mainly carried by the domains rigor, bradykinesia, and gait and persisted after 1 month (p = 0.009), whereas tremor remained unchanged.
Interpretation: The effect of a high-dose transcranial magnetic stimulation in patients with Parkinson's disease is encouraging and comparable to other studies using much longer stimulation protocols. This short-term intervention of 5 days facilitates the future application in an outpatient setting. Reduction in hospitalization rates directly benefits patients with motor impairment.
© 2024 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
MGE received research support from the German Ministry of Education and Research (BMBF) within the European Joint Program for Rare Diseases (EJP‐RD) 2021 Transnational Call for Rare Disease Research Projects (funding number 01GM2110), from the National Ataxia Foundation (NAF), and from Ataxia UK, and received consulting fees from Healthcare Manufaktur, Germany, all unrelated to this study. AF received travel support from CSL Behring, Ipsen, Ever Pharma and Indorsia, all unrelated to this project. AF and OK received research support from the German Parkinson's Association (Deutsche Parkinson Vereinigung), unrelated to this project. VB and TS declare no potential conflict of interest with respect to the research, the authorship and publication of this article.
Figures
References
-
- Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323:548‐560. - PubMed
-
- Gong C, Long Y, Peng X‐M, et al. Efficacy and safety of noninvasive brain stimulation for patients with cerebellar ataxia: a systematic review and meta‐analysis of randomized controlled trials. J Neurol. 2023;270:4782‐4799. - PubMed
-
- Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment‐resistant depression. Am J Psychiatry. 2020;177:716‐726. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

