Serum glial fibrillary acidic protein predicts disease progression in multiple sclerosis
- PMID: 39238198
- PMCID: PMC11514927
- DOI: 10.1002/acn3.52187
Serum glial fibrillary acidic protein predicts disease progression in multiple sclerosis
Abstract
Objective: Glial fibrillary acidic protein (GFAP) is expressed in astrocytes and may be a useful marker of non-active progressive multiple sclerosis (MS). We evaluate serum GFAP (sGFAP) in a large cohort of MS patients to determine if it predicts progression independent of relapse activity (PIRA), future gait aid, and conversion to secondary progressive disease (SPMS).
Methods: Adults with clinically isolated syndrome or any subtype of MS who were listed in the Brigham MS Center Research Database and had at least one sGFAP result were included. All clinic visits following first sample were analyzed for PIRA, future gait aid, and conversion to SPMS. Future cognitive dysfunction and fatigue were evaluated as secondary outcomes.
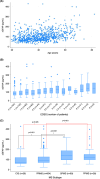
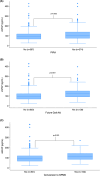
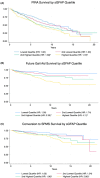
Results: In total, 741 patients were included (average age: 42.3, average disease duration: 3.7 years, median EDSS: 2, and median follow-up duration: 10.0 years). Of 643 patients (86.8%) without progressive disease at baseline, 15.9% developed SPMS. Among all 741, 50.5% had PIRA and 18.6% developed a gait aid requirement. sGFAP level predicted PIRA, future gait aid, and conversion to SPMS in univariable models (p < 0.001, <0.001, and 0.002). sGFAP remained predictive for PIRA and future gait aid in multivariable models in those younger than 50 (p = 0.048, 0.003). Change in sGFAP level over time was not predictive. There was no association between sGFAP and future fatigue or cognitive dysfunction.
Interpretation: sGFAP helps to predict PIRA, future gait aid, and conversion to SPMS in a large cohort of MS patients. Our data suggest that baseline levels may be more useful than the change over time.
© 2024 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
T.C. has received consulting fees and research support from Octave, which manufactures a commercial lab test for multiple serum biomarkers, including GFAP.
Figures

References
-
- Pittock SJ, McClelland RL, Mayr WT, et al. Clinical implications of benign multiple sclerosis: a 20‐year population‐based follow‐up study. Ann Neurol. 2004;56:303‐306. - PubMed
-
- Hirst C, Ingram G, Swingler R, Compston DA, Pickersgill T, Robertson NP. Change in disability in patients with multiple sclerosis: a 20‐year prospective population‐based analysis. J Neurol Neurosurg Psychiatry. 2008;79:1137‐1143. - PubMed
-
- Fambiatos A, Jokubaitis V, Horakova D, et al. Risk of secondary progressive multiple sclerosis: a longitudinal study. Mult Scler. 2020;26:79‐90. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

