Relative Rates of Transesterification vis-à-vis Newman's Rule of Six
- PMID: 39238420
- PMCID: PMC12272630
- DOI: 10.1021/acs.joc.4c01673
Relative Rates of Transesterification vis-à-vis Newman's Rule of Six
Abstract
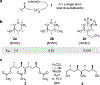
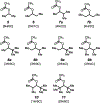
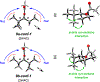
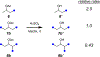
The relative reactivity of a systematic series of simple aliphatic acetate esters has been measured. Exposure of pairs of esters of increasing remote steric hindrance (by altering the degree of branching of the ester alkyl group) to a methanolic solution of Cs2CO3 proved to be a reliable (and general) method for quantitating the rate differences in these base-catalyzed transesterification reactions. The trends in relative rates are in accordance with the qualitative "Rule of Six" put forward by Melvin S. Newman in 1950, as deduced then from interpretation of earlier reports of ease of Fischer esterification reactions.
Conflict of interest statement
None of the authors have a competing financial interest in this work.
Figures

References
-
- Newman MS Some observations concerning steric factors. J. Am. Chem. Soc 1950, 72, 4783–4786.
-
- Bhide BV; Sudborough JJ Studies in transesterification. J. Ind. Inst. Sci 1925, 8, 89–128.
- von Braun J; Fischer F Beiträge zur Kenntnis der sterischen Hinderung, VII. Mitteil.: Veresterung und Verseifung vom Standpunkt der elektronischen Theorie der Bindung. Chem. Ber 1933, 66, 101–104.
- Smith HA; Burn J Kinetics of the acid-catalyzed esterification of phenyl- and cyclohexyl-substituted aliphatic acids in methanol. J. Am. Chem. Soc 1944, 66, 1494–1497.
-
- Newman MS Additions to unsaturated functions. In Steric Effects in Organic Chemistry; John Wiley & Sons, 1956; pp 201–248.
-
- This type of longer-range steric effect (or wrap-around effect) is similar to what is learned from the A-values in the monoalkylcyclohexanes (i.e., 1.7 Me to 1.8 Et to 2.2 iPr to ~5 tBu kcal mol−1); Anslyn EV; Dougherty DA Strain and stability. In Modern Physical Organic Chemistry; University Science, 2005; pp 65–143.
-
(b) The change of iPr to tBu introduces, perforce, a methyl carbon in place of a hydrogen that is six atoms removed from the axial hydrogen atoms at C3 and C5 of the cyclohexane ring.
-
-
A Google Scholar search (6–27-24) for “rule of six” within the 196 articles that cite reference #1 resulted in 61 hits, of which approximately half are in the context of polymer chemistry.
-
Grants and funding
LinkOut - more resources
Full Text Sources

