Capacitation of ram spermatozoa promotes changes in energy metabolism and aquaporin 3 and is affected by individual testosterone variations
- PMID: 39238428
- PMCID: PMC12006880
- DOI: 10.1111/andr.13756
Capacitation of ram spermatozoa promotes changes in energy metabolism and aquaporin 3 and is affected by individual testosterone variations
Abstract
Background: Recently, the metabolic pathways involved in energy production and the role of aquaglyceroporins in capacitation-associated events have been studied in humans and mice. However, little is known about these in ram spermatozoa.
Objective: The present study investigated bioenergetic and aquaglyceroporin 3 variations during in vitro capacitation of ram spermatozoa. In addition, differences in testosterone levels between males were examined to determine their influence on capacitation-like changes.
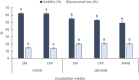
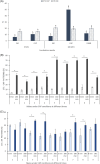
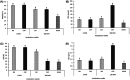
Materials and methods: Spermatozoa obtained from nine rams (ejaculates = 36) were incubated for 180 min in three different media (control, capacitating, and aquaglyceroporin-inhibitor media) at 38.5°C. At 0 and 180 min of incubation in each medium, sperm viability, kinetics, chlortetracycline patterns, adenosine triphosphate concentration, lactate excretion (final subproduct of glycolysis), and immunolocalization of aquaporin 3 were evaluated.
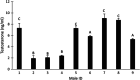
Results: The increment of the capacitated spermatozoa-chlortetracycline pattern and the hyperactivated-like movement characterized by the highest curvilinear velocity and amplitude of lateral head displacement and the lowest linearity was only recorded after 180 min in the capacitating medium. At this time and conditions, adenosine triphosphate content and lactate excretion decreased, whereas the aquaglyceroporin 3 location in the midpiece and principal piece increased compared to 0 min. Such changes were not observed in the control medium over time. Incubation in the aquaglyceroporin-inhibitor medium for 180 min reduced drastically sperm motility and adenosine triphosphate content compared to the other media. Testosterone analysis revealed a significant individual variability, which was also present in all sperm parameters evaluated. Furthermore, testosterone was negatively correlated with adenosine triphosphate content but positively correlated with lactate excretion levels, sperm viability, motility, capacitated sperm-chlortetracycline pattern, and aquaglyceroporin 3 immunolabeling in the midpiece and principal piece.
Conclusion: Despite individual differences, capacitation of ram spermatozoa increases adenosine triphosphate consumption, energy metabolism, and aquaglyceroporin 3 location in the midpiece and principal piece, which seems to be related to the acquisition of hyperactivated-like motility. Furthermore, testosterone levels may serve as a valuable tool to select those males with a greater sperm metabolism rate and fertilizing capacity.
Keywords: aquaglyceroporins; capacitation; hyperactivated motility; sperm metabolism; testosterone variations.
© 2024 The Author(s). Andrology published by John Wiley & Sons Ltd on behalf of American Society of Andrology and European Academy of Andrology.
Figures
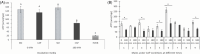


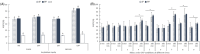

Similar articles
-
Specific order in the appearance of protein tyrosine phosphorylation patterns is functionally coordinated with dog sperm hyperactivation and capacitation.J Androl. 2003 May-Jun;24(3):423-37. doi: 10.1002/j.1939-4640.2003.tb02691.x. J Androl. 2003. PMID: 12721219
-
Energy Metabolism and Hyperactivation of Spermatozoa from Three Mouse Species under Capacitating Conditions.Cells. 2022 Jan 10;11(2):220. doi: 10.3390/cells11020220. Cells. 2022. PMID: 35053337 Free PMC article.
-
Glucose prevents the acquisition of the capacitated state in pig spermatozoa.Andrology. 2025 Mar;13(3):637-649. doi: 10.1111/andr.13691. Epub 2024 Jul 11. Andrology. 2025. PMID: 38993010 Free PMC article.
-
Redox regulation of mammalian sperm capacitation.Asian J Androl. 2015 Jul-Aug;17(4):583-90. doi: 10.4103/1008-682X.153303. Asian J Androl. 2015. PMID: 25926608 Free PMC article. Review.
-
Sperm Metabolism.Mol Reprod Dev. 2024 Oct;91(10):e23772. doi: 10.1002/mrd.23772. Mol Reprod Dev. 2024. PMID: 39407445 Review.
References
-
- Stival C, Molina CP, Paudel B, Buffone MG, Visconti PE, Krapf D. Sperm capacitation and acrosome reaction in mammalian sperm. In: Buffone MG, ed. Sperm Acrosome Biogenesis and Function During Fertilization. Springer; 2016:93‐106. - PubMed
-
- Freitas MJ, Vijayaraghavan S, Fardilha M. Signaling mechanisms in mammalian sperm motility. Biol Reprod. 2017;96(1):2‐12. - PubMed
-
- Leemans B, Stout TAE, De Schauwer C, et al. Update on mammalian sperm capacitation: how much does the horse differ from other species? Reproduction. 2019;157:181‐197. - PubMed
-
- Luna C, Serrano E, Domingo J, et al. Expression, cellular localization, and involvement of the pentose phosphate pathway enzymes in the regulation of ram sperm capacitation. Theriogenology. 2016;86(3):704‐714. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

