Exploring the potential of in vitro extracellular vesicle generation in reproductive biology
- PMID: 39238549
- PMCID: PMC11375532
- DOI: 10.1002/jex2.70007
Exploring the potential of in vitro extracellular vesicle generation in reproductive biology
Erratum in
-
Correction to "Exploring the Potential of in Vitro Extracellular Vesicle Generation in Reproductive Biology".J Extracell Biol. 2025 May 29;4(6):e70061. doi: 10.1002/jex2.70061. eCollection 2025 Jun. J Extracell Biol. 2025. PMID: 40453707 Free PMC article.
Abstract
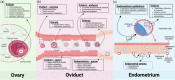
The interest in the growing field of extracellular vesicle (EV) research highlights their significance in intercellular signalling and the selective transfer of biological information between donor and recipient cells. EV studies have provided valuable insights into intercellular communication mechanisms, signal identification and their involvement in disease states, offering potential avenues for manipulating pathological conditions, detecting biomarkers and developing drug-delivery systems. While our understanding of EV functions in reproductive tissues has significantly progressed, exploring their potential as biomarkers for infertility, therapeutic interventions and enhancements in assisted reproductive technologies remains to be investigated. This knowledge gap stems partly from the difficulties associated with large-scale EV production relevant to clinical applications. Most existing studies on EV production rely on conventional 2D cell culture systems, characterized by suboptimal EV yields and a failure to replicate in vivo conditions. This results in the generation of EVs that differ from their in vivo counterparts. Hence, this review firstly delves into the importance of EVs in reproduction to then expand on current techniques for in vitro EV production, specifically examining diverse methods of culture and the potential of bioengineering technologies to establish innovative systems for enhanced EV production.
Keywords: bioengineering; improved EV yield; in vitro EV production.
© 2024 The Author(s). Journal of Extracellular Biology published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Abu‐Halima, M. , Häusler, S. , Backes, C. , Fehlmann, T. , Staib, C. , Nestel, S. , Nazarenko, I. , Meese, E. , & Keller, A. (2017). Micro‐ribonucleic acids and extracellular vesicles repertoire in the spent culture media is altered in women undergoing In Vitro Fertilization. Scientific Reports, 7(1), 13525. 10.1038/s41598-017-13683-8 - DOI - PMC - PubMed
-
- Adam, S. , Elfeky, O. , Kinhal, V. , Dutta, S. , Lai, A. , Jayabalan, N. , Nuzhat, Z. , Palma, C. , Rice, G. E. , & Salomon, C. (2017). Review: Fetal‐maternal communication via extracellular vesicles—Implications for complications of pregnancies. Placenta, 54, 83–88. 10.1016/j.placenta.2016.12.001 - DOI - PubMed
-
- Aguilera, C. , Velásquez, A. E. , Gutierrez‐Reinoso, M. A. , Wong, Y. S. , Melo‐Baez, B. , Cabezas, J. , Caamaño, D. , Navarrete, F. , Rojas, D. , Riadi, G. , Castro, F. O. , & Rodriguez‐Alvarez, L. (2023). Extracellular vesicles secreted by pre‐hatching bovine embryos produced in vitro and in vivo alter the expression of IFNtau‐stimulated genes in bovine endometrial cells. International Journal of Molecular Sciences, 24(8), 7438. 10.3390/ijms24087438 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
