Atezolizumab Plus Bevacizumab Treatment for Unresectable Hepatocellular Carcinoma: Real-life Experience from a Single Tertiary Centre in Spain and ALBI Score as a Survival Prognostic Factor
- PMID: 39238615
- PMCID: PMC11372697
- DOI: 10.21873/cdp.10373
Atezolizumab Plus Bevacizumab Treatment for Unresectable Hepatocellular Carcinoma: Real-life Experience from a Single Tertiary Centre in Spain and ALBI Score as a Survival Prognostic Factor
Abstract
Background/aim: Atezolizumab/bevacizumab (atez/bev) has been established as first-line systemic treatment for hepatocellular carcinoma (HCC). However, concerns regarding safety and efficacy have been raised, and no biomarkers to predict response have yet been identified. We aimed to evaluate the real-life experience of atez/bev in a Spanish tertiary hospital and identify factors associated with overall survival (OS).
Patients and methods: A prospective study of consecutive patients with HCC treated with atez/bev was conducted from December 2020 to December 2022. Efficacy was assessed through OS and progression-free survival (PFS), whereas safety was evaluated based on adverse events (AE). Twenty-three patients were included; 91% were males with a mean of 70 years. Thirteen patients were classified as having BCLC-C.
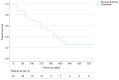
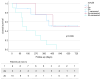
Results: The median treatment duration was 126 days (range=567). Median OS was 381 days (95%CI=205-557) with a cumulative probability of death of 13%, 30%, and 49% at 3, 6 and 12-month follow-up, respectively. The only factor associated with OS was the ALBI score (HR=5.03; 95%CI=1.3-19.1), which showed an AUROC of 0.906 (95%CI=0.78-1.00) for the risk of death at 18 months follow up. Median PFS was 141 days (95%CI=110-172). Twenty (86.9%) patients experienced AE, which in nine (39.1%) cases led to the definitive discontinuation of the treatment, four of them (17.4%) due to an AE grade 5.
Conclusion: The initial experience with atez/bev at our center demonstrated poorer outcomes compared to the original trial (IMbrave150). A careful assessment of the ALBI score may serve as a crucial factor in the selection of systemic treatment for patients with HCC.
Keywords: Hepatocellular carcinoma; adverse events; atezolizumab; bevacizumab; prognostic factors; real-world.
©2024 The Author(s). Published by the International Institute of Anticancer Research.
Conflict of interest statement
The Authors have no conflicts of interest to declare.
Figures
References
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. - DOI - PubMed
-
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL, IMbrave150 Investigators Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. - DOI - PubMed
-
- Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018. - DOI - PMC - PubMed
-
- Hiraoka A, Kumada T, Tada T, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Ogawa C, Nishimura T, Hatanaka T, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Koizumi Y, Nakamura S, Joko K, Iijima H, Hiasa Y, Kudo M, Real-life Practice Experts for HCC (RELPEC) Study Group, HCC 48 Group (Hepatocellular Carcinoma Experts from 48 Clinics in Japan) Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: Early clinical experience. Cancer Rep (Hoboken) 2022;5(2):e1464. doi: 10.1002/cnr2.1464. - DOI - PMC - PubMed
-
- Sho T, Suda G, Ogawa K, Kimura M, Kubo A, Tokuchi Y, Kitagataya T, Maehara O, Ohnishi S, Shigesawa T, Nakamura A, Yamada R, Ohara M, Kawagishi N, Natsuizaka M, Nakai M, Morikawa K, Furuya K, Baba M, Yamamoto Y, Suzuki K, Izumi T, Meguro T, Terashita K, Ito J, Miyagishima T, Sakamoto N. Early response and safety of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in patients who do not meet IMbrave150 eligibility criteria. Hepatol Res. 2021;51(9):979–989. doi: 10.1111/hepr.13693. - DOI - PubMed
LinkOut - more resources
Full Text Sources