Single-domain antibodies and aptamers drive new opportunities for neurodegenerative disease research
- PMID: 39238639
- PMCID: PMC11374656
- DOI: 10.3389/fimmu.2024.1426656
Single-domain antibodies and aptamers drive new opportunities for neurodegenerative disease research
Abstract
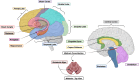
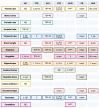
Neurodegenerative diseases (NDs) in mammals, such as Alzheimer's disease (AD), Parkinson's disease (PD), and transmissible spongiform encephalopathies (TSEs), are characterized by the accumulation of misfolded proteins in the central nervous system (CNS). Despite the presence of these pathogenic proteins, the immune response in affected individuals remains notably muted. Traditional immunological strategies, particularly those reliant on monoclonal antibodies (mAbs), face challenges related to tissue penetration, blood-brain barrier (BBB) crossing, and maintaining protein stability. This has led to a burgeoning interest in alternative immunotherapeutic avenues. Notably, single-domain antibodies (or nanobodies) and aptamers have emerged as promising candidates, as their reduced size facilitates high affinity antigen binding and they exhibit superior biophysical stability compared to mAbs. Aptamers, synthetic molecules generated from DNA or RNA ligands, present both rapid production times and cost-effective solutions. Both nanobodies and aptamers exhibit inherent qualities suitable for ND research and therapeutic development. Cross-seeding events must be considered in both traditional and small-molecule-based immunodiagnostic and therapeutic approaches, as well as subsequent neurotoxic impacts and complications beyond protein aggregates. This review delineates the challenges traditional immunological methods pose in ND research and underscores the potential of nanobodies and aptamers in advancing next-generation ND diagnostics and therapeutics.
Keywords: Alzheimer’s disease; Parkinson’s disease; nanobody; prion disease; therapeutics; transmissible spongiform encephalopathy.
Copyright © 2024 Shoemaker, Larsen and Larsen.
Conflict of interest statement
PL is a co-founder and stock owner of Priogen Corp, a diagnostic company specializing in the ultra-sensitive detection of pathogenic proteins associated with prion and protein-misfolding diseases. RL is an employee of Priogen Corp. The University of Minnesota licensed patent applications to Priogen Corp.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

