Advanced Dental Care: β-Chitosan Zinc Oxide Nanoparticles Targeting Cariogenic Microorganisms
- PMID: 39238748
- PMCID: PMC11376470
- DOI: 10.7759/cureus.66296
Advanced Dental Care: β-Chitosan Zinc Oxide Nanoparticles Targeting Cariogenic Microorganisms
Abstract
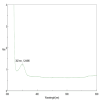
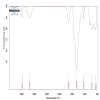
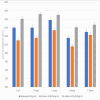
Introduction Dental caries, primarily caused by cariogenic microorganisms, remains a significant global health concern. β-Chitosan, known for its biofilm-targeting properties, and zinc oxide (ZnO) nanoparticles (NPs), recognized for their potent antimicrobial effects, offer a promising approach for caries prevention and treatment. This study investigates the synthesis, characterization, and antimicrobial properties of β-Chitosan-derived ZnO NPs (β-Ch-ZnO-NPs) against these pathogens. Methodology β-Chitosan from fresh squid bones was isolated using demineralization and deproteinization methods. β-Ch-ZnO-NPs were synthesized and characterized using UV-vis spectroscopy and Fourier-transform infrared spectroscopy (FTIR) to confirm their size, shape, and stability. Antibacterial efficacy(agar well plate method)was assessed through standardized assays, demonstrating significant inhibition of cariogenic bacteria. The results were represented as mean± standard deviation. The Kruskal-Wallis test with post hoc analysis (Mann-Whitney U test) was conducted for statistical analysis. Molecular docking studies (blind docking method) were conducted to elucidate the interactions between β-Ch-ZnO-NPs and key bacterial enzymes involved in microbial genetic material synthesis, also known as dihydroorotate dehydrogenase (DHODH, PDB ID-2J0Y). Results The synthesized β-Ch-ZnO-NPs exhibited well-defined characteristics verified by UV-vis spectroscopy and FTIR confirming their nanoparticulate nature and stability. The antimicrobial effects of Streptomycin (50 µg/mL) and β-Ch-ZnO-NPs were compared across various microorganisms. β-Ch-ZnO-NPs at 100 µg/mL consistently showed larger inhibition zones than Streptomycin and β-Ch-ZnO-NPs at 50 µg/mL against Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, Streptococcus mutans, and Candida albicans.This suggests that β-Ch-ZnO-NPs at a higher concentration have potent antimicrobial activity across a broad spectrum of pathogens, highlighting their potential as effective antimicrobial agents. Kruskal-Wallis test showed statistically significant differences (P < 0.001) for all microbes, and post hoc analysis (Mann-Whitney U test) confirmed the P-value was less than 0.05. Molecular docking studies indicated strong binding affinities between β-Ch-ZnO-NPs and bacterial enzymes crucial for biofilm formation, suggesting inhibition of enzyme activity critical for bacterial virulence and survival. Conclusions This study highlights the synergistic potential of β-Chitosan and zinc oxide NPs in combating dental caries. The synthesized β-Ch-ZnO-NPs demonstrated effective antimicrobial activity against cariogenic microorganisms, attributed to their ability to disrupt bacterial metabolism and inhibit biofilm formation. Molecular docking analysis provided mechanistic insights into how β-Ch-ZnO-NPs interact with bacterial enzymes, reinforcing their role in impeding biofilm development. Overall, the findings support using β-Ch-ZnO-NPs as a promising therapeutic strategy for preventing and treating dental caries, leveraging their combined biofilm-targeting capabilities and antimicrobial effects.
Keywords: antimicrobial properties; bacterial enzymes; biofilm inhibition; dental caries; molecular docking; β-chitosan-derived zinc oxide nanoparticles.
Copyright © 2024, Rajasekar et al.
Conflict of interest statement
Human subjects: All authors have confirmed that this study did not involve human participants or tissue. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- Beyond Streptococcus mutans: clinical implications of the evolving dental caries aetiological paradigms and its associated microbiome. Philip N, Suneja B, Walsh L. Br Dent J. 2018;224:219–225. - PubMed
-
- Dental caries. Pitts NB, Zero DT, Marsh PD, et al. Nat Rev Dis Primers. 2017;3:17030. - PubMed
-
- Caries management pathways preserve dental tissues and promote oral health. Ismail AI, Tellez M, Pitts NB, et al. Community Dent Oral Epidemiol. 2013;41:0–40. - PubMed
LinkOut - more resources
Full Text Sources
