Real-world outcomes of single-stage spinal cord stimulation in chronic pain patients: A multicentre, European case series
- PMID: 39238903
- PMCID: PMC11372901
- DOI: 10.1016/j.inpm.2023.100263
Real-world outcomes of single-stage spinal cord stimulation in chronic pain patients: A multicentre, European case series
Abstract
Background: Spinal cord stimulation (SCS) is effective in treating chronic neuropathic pain. A screening trial is typically conducted prior to implantation to evaluate whether a patient is a good candidate for SCS. However, the need for a screening trial has been debated. We evaluated real-world clinical outcomes in patients who underwent a single-stage procedure to receive SCS therapy (i.e., no screening trial period) (SS-SCS).
Methods: This observational, multicentre, real-world consecutive case series evaluated SS-SCS chronic pain patients. Pain and other functional outcomes were collected as part of standard care by site personnel with no sponsor involvement. Assessments included Numerical rating scale (NRS), Percent Pain Relief (PPR) and EQ-5D-5L (EuroQol 5 Dimensions-5L), recorded prior to SCS and following implantation.
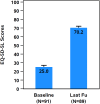
Results: A total of 171 chronic pain patients (mean age: 59.4; 53.2% females) underwent a single-stage procedure (mean last follow-up, 408 days) and were included in the analysis. A 5.0 ± 2.1-point improvement in overall pain was reported at 3 months and sustained until the last follow-up post-implantation (p < 0.0001). At last follow-up, 50.3% (86/171) of patients reported an NRS pain score ≤3. Additionally, quality of life also improved (46.1-point change, from 70.2 to 25) at the last follow-up, based on EQ-5D-5L scores.
Conclusions: In routine clinical practice, SS-SCS can provide significant long-term pain relief and improve quality of life in chronic pain patients. Our results suggest that effective long-term outcomes and success may be achieved without a trial period prior to permanent implantation of an SCS system.
Keywords: Chronic pain; Neuromodulation; Single-stage procedure; Spinal cord stimulation; Trial screening.
© 2023 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Pasquale De Negri reports administrative support, statistical analysis, and writing assistance were provided by Boston Scientific Neuromodulation. Pasquale De Negri reports a relationship with Boston Scientific Neuromodulation that includes: non-financial support. This study was sponsored by 10.13039/100008497Boston Scientific, and personnel from the Clinical Research Department within Boston Scientific’s Division of Neuromodulation were involved in the study design (RJ), analysis (RJ, YP), interpretation of the data (RJ), writing of the manuscript (RJ) and overall decision to submit the article for publication. 10.13039/100008497Boston Scientific also funded the services of the medical writer (Deborah Nock, Medical WriteAway, Norwich, UK). Drs. Jose F. Paz-Solis, Philippe Rigoard, Sylvie Raoul, Simon Thomson and Georgios K. Matis declare active consulting agreements with Boston Scientific. Dr. Abejón is a consultant and speaker for Boston Scientific, Saluda Médical, Medtronic and Abbott, Devonlabs, Cardiva2 and Grünenthal. Yu Pei and Roshini Jain are employees of Boston Scientific. This study is sponsored by 10.13039/100008497Boston Scientific.
Figures




References
-
- Fatima K., Javed S.O., Saleem A., Marsia S., Zafar R., Noorani K., Kumar S., Ali S.M., Ismail I., Hashim I., Ganatra F.A. Long-term efficacy of spinal cord stimulation for chronic primary neuropathic pain in the contemporary era: a systematic review and meta-analysis. J Neurosurg Sci. 2023 Mar 21 doi: 10.23736/S0390-5616.23.05930-1. Epub ahead of print. PMID: 36943763. - DOI - PubMed
-
- Eldabe S., Duarte R.V., Gulve A., Thomson S., Baranidharan G., Houten R., Jowett S., Sandhu H., Chadwick R., Brookes M., Kansal A., Earle J., Bell J., Robinson J., Walker S., Rhodes S., Taylor R.S. Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility and cost-effectiveness (TRIAL-STIM)? A randomised controlled trial. Pain. 2020 Dec;161(12):2820–2829. - PMC - PubMed
-
- Weinand M.E., Madhusudan H., Davis B., Melgar M. Acute vs. Prolonged screening for spinal cord stimulation in chronic pain. Neuromodulation. 2003;6(1):15–19. https://10.1046/j.1525-1403.2003.03002.x - DOI - PubMed
-
- Oakley J.C., Krames E.S., Stamatos J., Foster A.M. Successful long-term outcomes of spinal cord stimulation despite limited pain relief during temporary trialing. Neuromodulation. 2008;11(1):66–73. https://10.1111/j.1525-1403.2007.00145.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

