Exploring senescence as a modifier of β cell extracellular vesicles in type 1 diabetes
- PMID: 39239092
- PMCID: PMC11374605
- DOI: 10.3389/fendo.2024.1422279
Exploring senescence as a modifier of β cell extracellular vesicles in type 1 diabetes
Abstract
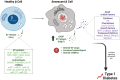
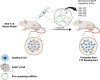
Type 1 Diabetes (T1D) is a chronic metabolic disease resulting from insulin deficiency due to autoimmune loss of pancreatic β cells. In addition to β cell destruction, it is now accepted that β cell stress and dysfunction, such as senescence, plays a crucial role in the development of the disease. Accumulation of senescent β cells occurs during development of T1D in humans and contributes to the progression of T1D in the nonobese diabetic (NOD) mouse model. Senescent β cells are thought to exacerbate the inflammatory response within the islets by production and secretion of senescence-associated secretory phenotype (SASP). Extracellular vesicles (EVs) from β cells have been shown to carry protein and microRNAs (miRNAs), influencing cellular signaling and may contribute to the development of T1D but it remains to be addressed how senescence impacts β cell EV cargo. In this minireview, we discuss emerging evidence that EV cargo proteins and miRNAs associated with senescence could contribute to the development of T1D and could suggest potential biomarkers and therapeutic targets for the regulation of SASP and elimination of senescent β cells in T1D. Future investigation exploring the intricate relationship between β cell senescence, EVs and miRNAs could pave the way for the development of novel diagnostic techniques and therapeutic interventions.
Keywords: SASP (senescence-associated secretory phenotype); cellular senescence; extracellular vesicles; microRNAs; type 1 diabetes.
Copyright © 2024 Motlagh, Pipella and Thompson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

