Mechanotransduction of the vasculature in Hutchinson-Gilford Progeria Syndrome
- PMID: 39239311
- PMCID: PMC11374724
- DOI: 10.3389/fphys.2024.1464678
Mechanotransduction of the vasculature in Hutchinson-Gilford Progeria Syndrome
Abstract
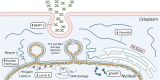
Hutchinson-Gilford Progeria Syndrome (HGPS) is a premature aging disorder that causes severe cardiovascular disease, resulting in the death of patients in their teenage years. The disease pathology is caused by the accumulation of progerin, a mutated form of the nuclear lamina protein, lamin A. Progerin binds to the inner nuclear membrane, disrupting nuclear integrity, and causes severe nuclear abnormalities and changes in gene expression. This results in increased cellular inflammation, senescence, and overall dysfunction. The molecular mechanisms by which progerin induces the disease pathology are not fully understood. Progerin's detrimental impact on nuclear mechanics and the role of the nucleus as a mechanosensor suggests dysfunctional mechanotransduction could play a role in HGPS. This is especially relevant in cells exposed to dynamic, continuous mechanical stimuli, like those of the vasculature. The endothelial (ECs) and smooth muscle cells (SMCs) within arteries rely on physical forces produced by blood flow to maintain function and homeostasis. Certain regions within arteries produce disturbed flow, leading to an impaired transduction of mechanical signals, and a reduction in cellular function, which also occurs in HGPS. In this review, we discuss the mechanics of nuclear mechanotransduction, how this is disrupted in HGPS, and what effect this has on cell health and function. We also address healthy responses of ECs and SMCs to physiological mechanical stimuli and how these responses are impaired by progerin accumulation.
Keywords: atherosclerosis; endothelial cell; lamin a; mechanotransduction; progeria; progerin; vascular smooth muscle cell.
Copyright © 2024 Shores and Truskey.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Angiopoietin-2 reverses endothelial cell dysfunction in progeria vasculature.Aging Cell. 2025 Feb;24(2):e14375. doi: 10.1111/acel.14375. Epub 2024 Oct 18. Aging Cell. 2025. PMID: 39422121 Free PMC article.
-
The Accumulation of Progerin Underlies the Loss of Aortic Smooth Muscle Cells in Hutchinson-Gilford Progeria Syndrome.bioRxiv [Preprint]. 2024 Nov 3:2024.10.29.620896. doi: 10.1101/2024.10.29.620896. bioRxiv. 2024. Update in: Cell Death Dis. 2025 Jul 24;16(1):557. doi: 10.1038/s41419-025-07853-0. PMID: 39554077 Free PMC article. Updated. Preprint.
-
The anti-senescence effect of D-β-hydroxybutyrate in Hutchinson-Gilford progeria syndrome involves progerin clearance by the activation of the AMPK-mTOR-autophagy pathway.Geroscience. 2025 Jun;47(3):3849-3871. doi: 10.1007/s11357-024-01501-9. Epub 2025 Jan 16. Geroscience. 2025. PMID: 39821043 Free PMC article.
-
From gene to mechanics: a comprehensive insight into the mechanobiology of LMNA mutations in cardiomyopathy.Cell Commun Signal. 2024 Mar 27;22(1):197. doi: 10.1186/s12964-024-01546-5. Cell Commun Signal. 2024. PMID: 38539233 Free PMC article. Review.
-
Uncommon Non-MS Demyelinating Disorders of the Central Nervous System.Curr Neurol Neurosci Rep. 2025 Jul 1;25(1):45. doi: 10.1007/s11910-025-01432-8. Curr Neurol Neurosci Rep. 2025. PMID: 40591029 Review.
Cited by
-
The accumulation of progerin underlies the loss of aortic smooth muscle cells in Hutchinson-Gilford progeria syndrome.Cell Death Dis. 2025 Jul 24;16(1):557. doi: 10.1038/s41419-025-07853-0. Cell Death Dis. 2025. PMID: 40707465 Free PMC article.
References
-
- Atchison L., Abutaleb N. O., Snyder-Mounts E., Gete Y., Ladha A., Ribar T., et al. (2020). iPSC-derived endothelial cells affect vascular function in a tissue-engineered blood vessel model of hutchinson-gilford progeria syndrome. Stem Cell Rep. 14, 325–337. 10.1016/j.stemcr.2020.01.005 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

