Cumulative glucocorticoid exposure in patients receiving epidural steroid injections: A single-centre retrospective evaluation on 581 procedures against existing clinical recommendations
- PMID: 39239375
- PMCID: PMC11372952
- DOI: 10.1016/j.inpm.2022.100094
Cumulative glucocorticoid exposure in patients receiving epidural steroid injections: A single-centre retrospective evaluation on 581 procedures against existing clinical recommendations
Abstract
Background: The purpose of the study was to review the cumulative corticosteroid doses received from epidural and non-epidural-based pain interventions in a cohort of patients undergoing epidural steroid injections (ESIs) with comparison to safe dosing recommendations.
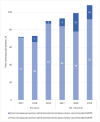
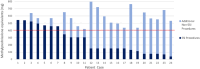
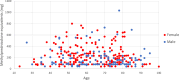
Methods: Retrospective analysis was undertaken for all 349 patients who underwent a total of 581 ESIs at a single-centre, tertiary hospital in South Australia between 2017 and 2019. The primary outcome was the yearly dose analysis of cumulative steroid doses in methylprednisolone equivalents (MDPE) administered from epidural and non-epidural interventions in post-menopausal women, interpreted against maximum recommended doses.
Results: The annual limit of 200 mg for postmenopausal women was exceeded in 4.7% of the time (11/235) from ESIs alone, with a significant rise to 15.3% (46/300) when non-ESI injections were included in cumulative dose totals(p < 0.001). Of the 173 participants of post-menopausal female age, 4.1% (7/173) received cumulative corticosteroid doses above the 3-year 400 mg MPDE limit from ESIs alone, with a statistically significant increase to 13.9% (24/173) when non-epidural steroid injections were again included in cumulative dose totals (p < 0.001). The mean ± standard deviation administered MPDE per epidural steroid injection across the whole study cohort was 72 ± 22 mg, nearly double the recommended dose of 40 mg.
Conclusions: Our study underpins the need for vigilance when considering steroid-based pain interventions, wherein both the individual and cumulative steroid exposure should be considered.
Keywords: Epidural pain interventions; Glucocorticoids; Non-epidural pain interventions; Postmenopausal women; Steroid dose exposure.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Jamison D.E., Cohen S.P. Critically evaluating the evidence for epidural injections for failed back surgery syndrome: should pain physicians Be bracing for impact? Pain Med. 2018;19(7):1299–1301. - PubMed
-
- Harris I.A., Buchbinder R. Time to reconsider steroid injections in the spine? Med J Aust. 2013;25(8):629–633. - PubMed
LinkOut - more resources
Full Text Sources

