Coconut milk allergenicity: Insight into reducing the affinity of coconut globulin to immunoglobulin E by atmospheric cold plasma
- PMID: 39239533
- PMCID: PMC11375244
- DOI: 10.1016/j.fochx.2024.101732
Coconut milk allergenicity: Insight into reducing the affinity of coconut globulin to immunoglobulin E by atmospheric cold plasma
Abstract
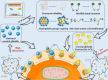
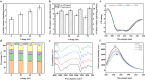
Atmospheric cold plasma (ACP) presents a promising method for the sterilization of coconut milk and exhibits a modifying effect on coconut globulin (CG), the primary allergen in coconut milk. This study investigated the potential role of ACP treatment in mitigating the allergenic properties of coconut milk by examining changes in protein structure. ACP treatment induced structural alterations in CG, disrupting binding sites with immunoglobulin E (IgE). Consequently, this led to a reduction in the affinity between CG and IgE, evidenced by a decrease in Ka from 2.17 × 104/M to 0.64 × 104/M, thereby diminishing IgE-mediated allergic reactions. The findings from allergenic and cellular models further corroborated that ACP treatment decreased the allergenicity of CG by 55.18%, while inhibiting degranulation and the release of allergic mediators. This study presents an innovative methodology for producing hypoallergenic coconut milk, thereby expanding the applicability of ACP technology within the food industry.
Keywords: Affinity; Allergenicity reduction; Binding capacity; Hydrophobic group; Spatial structure.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Coconut milk treated by atmospheric cold plasma: Effect on quality and stability.Food Chem. 2024 Jan 1;430:137045. doi: 10.1016/j.foodchem.2023.137045. Epub 2023 Jul 28. Food Chem. 2024. PMID: 37541035
-
Investigating binding mechanism between coconut globulin and tannic acid mediated by atmospheric cold plasma: Protein structure and stability.Food Chem. 2025 Feb 1;464(Pt 2):141670. doi: 10.1016/j.foodchem.2024.141670. Epub 2024 Oct 18. Food Chem. 2025. PMID: 39432945
-
Evaluating the influence of cold plasma bubbling on protein structure and allergenicity in sesame milk.Allergol Immunopathol (Madr). 2023 Mar 13;51(S Pt 1):1-13. doi: 10.15586/aei.v51iSP1.783. eCollection 2023. Allergol Immunopathol (Madr). 2023. PMID: 36924386
-
Why are some proteins allergenic? Implications for biotechnology.Crit Rev Food Sci Nutr. 1996 Jul;36(6):553-64. doi: 10.1080/10408399609527739. Crit Rev Food Sci Nutr. 1996. PMID: 8841731 Review.
-
The composition, extraction, functional property, quality, and health benefits of coconut protein: A review.Int J Biol Macromol. 2024 Nov;280(Pt 4):135905. doi: 10.1016/j.ijbiomac.2024.135905. Epub 2024 Sep 25. Int J Biol Macromol. 2024. PMID: 39332551 Review.
Cited by
-
Traditional and Emerging Physical Processing Technologies: Applications and Challenges in Allergen Control of Animal and Plant Proteins.Compr Rev Food Sci Food Saf. 2025 Jul;24(4):e70196. doi: 10.1111/1541-4337.70196. Compr Rev Food Sci Food Saf. 2025. PMID: 40519066 Free PMC article. Review.
References
-
- Benede S., Lozano-Ojalvo D., Cristobal S., Costa J., D’Auria E., Velickovic T.C., et al. New applications of advanced instrumental techniques for the characterization of food allergenic proteins. Critical Reviews in Food Science and Nutrition. 2022;62(31):8686–8702. doi: 10.1080/10408398.2021.1931806. - DOI - PubMed
-
- Chen Y., Chen Y.L., Jiang L.Z., Yang Z.H., Fang Y.J., Zhang W.M. Shear emulsification condition strategy impact high internal phase Pickering emulsions stabilized by coconut globulin-tannic acid: Structure of protein at the oil-water interface. LWT - Food Science and Technology. 2023;187:115283. doi: 10.1016/j.lwt.2023.115283. - DOI
-
- Chen Y., Yao M.Y., Yang T.Y., Fang Y.J., Xiang D., Zhang W.M. Changes in structure and emulsifying properties of coconut globulin after the atmospheric pressure cold plasma treatment. Food Hydrocolloids. 2023;136 doi: 10.1016/j.foodhyd.2022.108289. - DOI
LinkOut - more resources
Full Text Sources

