A systematic review of normal tissue neurovascular unit damage following brain irradiation-Factors affecting damage severity and timing of effects
- PMID: 39239570
- PMCID: PMC11375288
- DOI: 10.1093/noajnl/vdae098
A systematic review of normal tissue neurovascular unit damage following brain irradiation-Factors affecting damage severity and timing of effects
Abstract
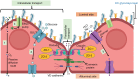
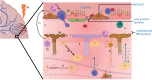
Background: Radiotherapy is key in the treatment of primary and secondary brain tumors. However, normal tissue is inevitably irradiated, causing toxicity and contributing to cognitive dysfunction. The relative importance of vascular damage to cognitive decline is poorly understood. Here, we systematically review the evidence for radiation-induced damage to the entire neurovascular unit (NVU), particularly focusing on establishing the factors that influence damage severity, and timing and duration of vascular effects relative to effects on neural tissue.
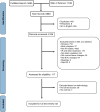
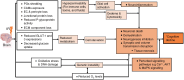
Methods: Using PubMed and Web of Science, we searched preclinical and clinical literature published between January 1, 1970 and December 1, 2022 and evaluated factors influencing NVU damage severity and timing of NVU effects resulting from ionizing radiation.
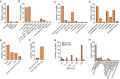
Results: Seventy-two rodents, 4 canines, 1 rabbit, and 5 human studies met inclusion criteria. Radiation increased blood-brain barrier (BBB) permeability, reduced endothelial cell number and extracellular matrix proteoglycans, reduced tight junction proteins, upregulated cellular adhesion molecule expression, reduced activity of glucose and BBB efflux transporters and activated glial cells. In the brain parenchyma, increased metalloproteinases 2 and 9 levels, demyelination, cell death, and inhibited differentiation were observed. Effects on the vasculature and neural compartment were observed across acute, delayed, and late timepoints, and damage extent was higher with low linear energy transfer radiation, higher doses, lower dose rates, broader beams, and in the presence of a tumor.
Conclusions: Irradiation of normal brain tissue leads to widespread and varied impacts on the NVU. Data indicate that vascular damage is in most cases an early effect that does not quickly resolve. More studies are needed to confirm sequence of damages, and mechanisms that lead to cognitive dysfunction.
Keywords: blood-brain barrier damage; brain irradiation; cognitive decline; neurotoxicity; neurovascular unit dysfunction.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
None declared.
Figures

Similar articles
-
Modeling ischemic stroke in a triculture neurovascular unit on-a-chip.Fluids Barriers CNS. 2021 Dec 14;18(1):59. doi: 10.1186/s12987-021-00294-9. Fluids Barriers CNS. 2021. PMID: 34906183 Free PMC article.
-
Profiling the neurovascular unit unveils detrimental effects of osteopontin on the blood-brain barrier in acute ischemic stroke.Acta Neuropathol. 2022 Aug;144(2):305-337. doi: 10.1007/s00401-022-02452-1. Epub 2022 Jun 25. Acta Neuropathol. 2022. PMID: 35752654 Free PMC article.
-
The "Neuro-Glial-Vascular" Unit: The Role of Glia in Neurovascular Unit Formation and Dysfunction.Front Cell Dev Biol. 2021 Sep 27;9:732820. doi: 10.3389/fcell.2021.732820. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34646826 Free PMC article. Review.
-
Stroke-induced damage on the blood-brain barrier.Front Neurol. 2023 Sep 28;14:1248970. doi: 10.3389/fneur.2023.1248970. eCollection 2023. Front Neurol. 2023. PMID: 37840921 Free PMC article. Review.
-
Neurological diseases in relation to the blood-brain barrier.J Cereb Blood Flow Metab. 2012 Jul;32(7):1139-51. doi: 10.1038/jcbfm.2011.197. Epub 2012 Jan 18. J Cereb Blood Flow Metab. 2012. PMID: 22252235 Free PMC article. Review.
Cited by
-
Multiple Sclerosis-like Lesions Induced by Radiation: A Case Report and Systematic Review of the Literature.J Clin Med. 2024 Dec 12;13(24):7554. doi: 10.3390/jcm13247554. J Clin Med. 2024. PMID: 39768480 Free PMC article. Review.
-
Replacing 2 Gy Per Fraction Equivalent Dose with Fractionation-Specific Biological Equivalent Dose for Normal Tissues.Int J Mol Sci. 2024 Nov 30;25(23):12891. doi: 10.3390/ijms252312891. Int J Mol Sci. 2024. PMID: 39684602 Free PMC article.
-
Potential Risk of Cognitive Impairment Due to Irradiation of Neural Structures in Locally Advanced Nasopharyngeal Cancer Treated by Curative Radiotherapy.Medicina (Kaunas). 2025 Apr 27;61(5):810. doi: 10.3390/medicina61050810. Medicina (Kaunas). 2025. PMID: 40428768 Free PMC article.
References
-
- Baumann M, Krause M, Overgaard J, et al.. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16(4):234–249. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
