Better cardiovascular health is associated with slowed clinical progression in autosomal dominant frontotemporal lobar degeneration variant carriers
- PMID: 39240048
- PMCID: PMC11485313
- DOI: 10.1002/alz.14172
Better cardiovascular health is associated with slowed clinical progression in autosomal dominant frontotemporal lobar degeneration variant carriers
Abstract
Introduction: Cardiovascular health is important for brain aging, yet its role in the clinical manifestation of autosomal dominant or atypical forms of dementia has not been fully elucidated. We examined relationships between Life's Simple 7 (LS7) and clinical trajectories in individuals with autosomal dominant frontotemporal lobar degeneration (FTLD).
Methods: Two hundred forty-seven adults carrying FTLD pathogenic genetic variants (53% asymptomatic) and 189 non-carrier controls completed baseline LS7, and longitudinal neuroimaging and neuropsychological testing.
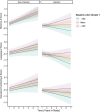
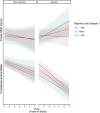
Results: Among variant carriers, higher baseline LS7 is associated with slower accumulation of frontal white matter hyperintensities (WMHs), as well as slower memory and language declines. Higher baseline LS7 associated with larger baseline frontotemporal volume, but not frontotemporal volume trajectories.
Discussion: Better baseline cardiovascular health related to slower cognitive decline and accumulation of frontal WMHs in autosomal dominant FTLD. Optimizing cardiovascular health may be an important modifiable approach to bolster cognitive health and brain integrity in FTLD.
Highlights: Better cardiovascular health associates with slower cognitive decline in frontotemporal lobar degeneration (FTLD). Lifestyle relates to the accumulation of frontal white matter hyperintensities in FTLD. More optimal cardiovascular health associates with greater baseline frontotemporal lobe volume. Optimized cardiovascular health relates to more favorable outcomes in genetic dementia.
Keywords: Life's Simple 7; aging; cardiovascular health; frontotemporal dementia; genetic dementia; lifestyle behaviors; modifiable risk; neuropsychology.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Anna M. VandeBunte, Hyunwoo Lee, Emily W. Paolillo, Rowan Saloner, Katya Rascovsky, Allison Snyder, Mario F. Mendez, Ryan Darby, Hilary Heuer, and Kaitlin B. Casaletto have nothing to disclose. Ging‐Yuek Robin Hsiung received grant support from CIHR, NIH, and Alzheimer Society of BC; participated in clinical trials sponsored by Anavax, Biogen, Cassava, Eli Lilly, and Roche; and served as a consultant to Biogen, Novo Nordisk, and Roche. Adam Staffaroni is a co‐inventor of four ALLFTD mApp tasks and receives licensing fees. Datacubed Health was not involved with the analysis or reporting of study data. He has also received research support from the NIA/NIH, Bluefield Project to Cure FTD, the Alzheimer's Association, the Larry L. Hillblom Foundation, the Rainwater Charitable Foundation, and has provided consultation to Alector, Lilly/Prevail, Passage Bio, and Takeda. Carmela Tartaglia has served as an investigator for clinical trials sponsored by Biogen, Avanex, Green Valley, Roche/Genentech, Bristol Myers Squibb, Eli Lilly/Avid Radiopharmaceuticals, and Janssen. She receives research support from the Canadian Institutes of Health Research. Kristine Yaffe is on the board for Alector Inc. David S. Knopman serves on the data and safety monitoring board of the DIAN‐TU study; is a site principal investigator for clinical trials sponsored by Biogen, Lilly, and the University of Southern California; and is funded by the NIH. Eliana Marisa Ramos, Peter Pressman, and Julie A. Fields receive research support from NIH. Andrea C. Bozoki receives research funding from the NIH, Alector Inc., Cognition Therapeutics, EIP Pharma, and Transposon, Inc. She is a consultant for Eisai Pharmaceuticals and Creative Biopeptides and a member of the data safety monitoring board for AviadoBio. Bonnie Wong receives research support from the NIH. Kimiko Domoto‐Reilly receives research support from the NIH, and serves as an investigator for a clinical trial sponsored by Lawson Health Research Institute. Irene Litvan receives research support from NIH 1U19AG063911‐01 2R01AG038791‐06A, U01NS100610, U01NS80818, and 1R21NS114764‐01A1; the Michael J Fox Foundation, Parkinson Foundation, Lewy Body Association, CurePSP, Roche, Abbvie, Lundbeck, Novartis, Transposon, and UCB. She is a member of the scientific advisory board for the Rossy PSP Program at the University of Toronto, and of the scientific advisory board for Amydis but does not receive funds. She is the chief editor of
Figures

References
-
- Song R, Pan KY, Xu H, et al. Association of cardiovascular risk burden with risk of dementia and brain pathologies: a population‐based cohort study. Alzheimers Dement. 2021;17:1914‐1922. https://onlinelibrary.wiley.com/doi/full/10.1002/alz.12343 - DOI - PMC - PubMed
-
- Sheibani N, Wong KH, Turan TN, et al. White matter hyperintensity and cardiovascular disease outcomes in the SPRINT MIND trial. J Stroke Cerebrovasc Dis. 2021;30:105764. https://pmc/articles/PMC8107132/ - PMC - PubMed
-
- Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553‐561. https://jamanetwork.com/journals/jama/fullarticle/2723256 - PMC - PubMed
MeSH terms
Grants and funding
- U24 AG21886/ARTFL LEFFTDS Longitudinal Frontotemporal Lobar Degeneration
- U19AG063911/ARTFL LEFFTDS Longitudinal Frontotemporal Lobar Degeneration
- U01AG045390/Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects
- R56AG082414/ARTFL LEFFTDS Longitudinal Frontotemporal Lobar Degeneration
- P01AG019724/ARTFL LEFFTDS Longitudinal Frontotemporal Lobar Degeneration
- U54NS092089/Advancing Research and Treatment in Frontotemporal Lobar Degeneration
- U01 AG045390/AG/NIA NIH HHS/United States
- U54 NS092089/NS/NINDS NIH HHS/United States
- K23AG058752/ARTFL LEFFTDS Longitudinal Frontotemporal Lobar Degeneration
- NIA
- R01AG072475/ARTFL LEFFTDS Longitudinal Frontotemporal Lobar Degeneration
- R56 AG082414/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- U19 AG063911/AG/NIA NIH HHS/United States
- NIH
- P01 AG019724/AG/NIA NIH HHS/United States
- K23 AG058752/AG/NIA NIH HHS/United States
- R01 AG072475/AG/NIA NIH HHS/United States
- P30 AG066509/AG/NIA NIH HHS/United States

