Oral pharmacokinetics and efficacy of oral phospholipid remdesivir nucleoside prodrugs against SARS-CoV-2 in mice
- PMID: 39240093
- PMCID: PMC11459966
- DOI: 10.1128/aac.01039-24
Oral pharmacokinetics and efficacy of oral phospholipid remdesivir nucleoside prodrugs against SARS-CoV-2 in mice
Abstract
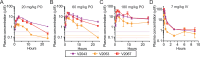
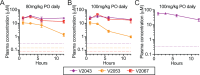
Oral broad-spectrum antivirals are urgently needed for the treatment of many emerging and contemporary RNA viruses. We previously synthesized 1-O-octadecyl-2-O-benzyl-sn-glyceryl-P-RVn (ODBG-P-RVn, V2043), a phospholipid prodrug of GS-441524 (remdesivir nucleoside, RVn), and demonstrated its in vivo efficacy in a SARS-CoV-2 mouse model. Structure-activity relationship studies focusing on the prodrug scaffold identified two modifications, 3-fluoro-4-methoxy-benzyl (V2053) and 4-cyano-benzyl (V2067), that significantly enhanced the in vitro broad-spectrum antiviral activity against multiple RNA viruses when compared to V2043. Here, we demonstrate that V2043, V2053, and V2067 are all orally bioavailable, well-tolerated, and achieve high sustained plasma levels after single oral daily dosing. All three phospholipid prodrugs are significantly more active than RVn in vitro and significantly reduce SARS-CoV-2 lung titers in prophylaxis and treatment mouse models of SARS-CoV-2 B.1.351 infection. On a molar basis, V2043 and V2067 are substantially more active than obeldesivir/GS-5245 and molnupiravir in vivo. Together, these data support the continued development of phospholipid RVn prodrugs for the treatment of SARS-CoV-2 and other RNA viruses of clinical concern.
Keywords: COVID-19; SARS-CoV-2; antiviral agent; broad spectrum antiviral; in vivo efficacy; lipid prodrug; mouse model; pharmacokinetics; remdesivir; remdesivir nucleoside.
Conflict of interest statement
K.Y.H., J.R.B., N.V., and R.T.S. are named as inventors of PCT/US2021/040394 and US 2023/0287029 (Antiviral Prodrugs, Pharmaceutical Formulations and Methods) and PCT/US2023/011693 (Antiviral Prodrugs, Intermediate and Long-Acting Formulations and Methods).
Figures



References
-
- Checkmahomed L, Carbonneau J, Du Pont V, Riola NC, Perry JK, Li J, Paré B, Simpson SM, Smith MA, Porter DP, Boivin G. 2022. In vitro selection of remdesivir-resistant SARS-CoV-2 demonstrates high barrier to resistance. Antimicrob Agents Chemother 66:e0019822. doi:10.1128/aac.00198-22 - DOI - PMC - PubMed
-
- Hedskog C, Rodriguez L, Roychoudhury P, Huang M-L, Jerome KR, Hao L, Ireton RC, Li J, Perry JK, Han D, Camus G, Greninger AL, Gale M, Porter DP. 2023. Viral resistance analyses from the remdesivir phase 3 adaptive COVID-19 treatment trial-1 (ACTT-1). J Infect Dis 228:1263–1273. doi:10.1093/infdis/jiad270 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

