Unfolding and de-confounding: biologically meaningful causal inference from longitudinal multi-omic networks using METALICA
- PMID: 39240096
- PMCID: PMC11494969
- DOI: 10.1128/msystems.01303-23
Unfolding and de-confounding: biologically meaningful causal inference from longitudinal multi-omic networks using METALICA
Abstract
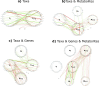
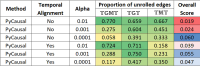
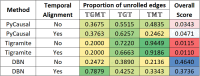
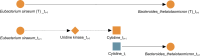
A key challenge in the analysis of microbiome data is the integration of multi-omic datasets and the discovery of interactions between microbial taxa, their expressed genes, and the metabolites they consume and/or produce. In an effort to improve the state of the art in inferring biologically meaningful multi-omic interactions, we sought to address some of the most fundamental issues in causal inference from longitudinal multi-omics microbiome data sets. We developed METALICA, a suite of tools and techniques that can infer interactions between microbiome entities. METALICA introduces novel unrolling and de-confounding techniques used to uncover multi-omic entities that are believed to act as confounders for some of the relationships that may be inferred using standard causal inferencing tools. The results lend support to predictions about biological models and processes by which microbial taxa interact with each other in a microbiome. The unrolling process helps identify putative intermediaries (genes and/or metabolites) to explain the interactions between microbes; the de-confounding process identifies putative common causes that may lead to spurious relationships to be inferred. METALICA was applied to the networks inferred by existing causal discovery, and network inference algorithms were applied to a multi-omics data set resulting from a longitudinal study of IBD microbiomes. The most significant unrollings and de-confoundings were manually validated using the existing literature and databases.
Importance: We have developed a suite of tools and techniques capable of inferring interactions between microbiome entities. METALICA introduces novel techniques called unrolling and de-confounding that are employed to uncover multi-omic entities considered to be confounders for some of the relationships that may be inferred using standard causal inferencing tools. To evaluate our method, we conducted tests on the inflammatory bowel disease (IBD) dataset from the iHMP longitudinal study, which we pre-processed in accordance with our previous work. From this dataset, we generated various subsets, encompassing different combinations of metagenomics, metabolomics, and metatranscriptomics datasets. Using these multi-omics datasets, we demonstrate how the unrolling process aids in the identification of putative intermediaries (genes and/or metabolites) to explain the interactions between microbes. Additionally, the de-confounding process identifies potential common causes that may give rise to spurious relationships to be inferred. The most significant unrollings and de-confoundings were manually validated using the existing literature and databases.
Keywords: causal inference; de-confounding; longitudinal microbiome analysis; multi-omic integration; unfolding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Update of
-
Unfolding and De-confounding: Biologically meaningful causal inference from longitudinal multi-omic networks using METALICA.bioRxiv [Preprint]. 2023 Dec 13:2023.12.12.571384. doi: 10.1101/2023.12.12.571384. bioRxiv. 2023. Update in: mSystems. 2024 Oct 22;9(10):e0130323. doi: 10.1128/msystems.01303-23. PMID: 38168315 Free PMC article. Updated. Preprint.
References
-
- Fernandez M, Aguiar-Pulido V, Riveros J, Huang W, Segal J, Zeng E, Campos M, Mathee K, Narasimhan G. 2016. Microbiome analysis: state of the art and future trends. Comput Methods for Next Gener Seq Data Anal:401–424. doi: 10.1002/9781119272182 - DOI
-
- Stebliankin V, Sazal M, Valdes C, Mathee K, Narasimhan G. 2022. A novel approach for combining the metagenome, metaresistome, metareplicome and causal inference to determine the microbes and their antibiotic resistance gene repertoire that contribute to dysbiosis. Microb Genom 8:mgen000899. doi: 10.1099/mgen.0.000899 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

