A cardioviral 2C-ATP complex structure reveals the essential role of a conserved arginine in regulation of cardioviral 2C activity
- PMID: 39240112
- PMCID: PMC11495053
- DOI: 10.1128/jvi.00911-24
A cardioviral 2C-ATP complex structure reveals the essential role of a conserved arginine in regulation of cardioviral 2C activity
Abstract
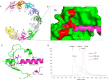
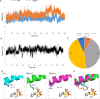
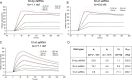
2C is a highly conserved picornaviral non-structural protein with ATPase activity and plays a multifunctional role in the viral life cycle as a promising target for anti-picornavirus drug development. While the structure-function of enteroviral 2Cs have been well studied, cardioviral 2Cs remain largely uncharacterized. Here, an endogenous ATP molecule was identified in the crystal structure of 2C from encephalomyocarditis virus (EMCV, Cardiovirus A). The ATP is bound into the ATPase active site with a unique compact conformation. Notably, the γ-phosphate of ATP directly interacts with Arg311 (conserved in cardioviral 2Cs), and its mutation significantly inhibits the ATPase activity. Unexpectedly, this mutation remarkably promotes 2C self-oligomerization and viral replication efficiency. Molecular dynamic simulations showed that the Arg311 side chain is highly dynamic, indicating it may function as a switch between the activation state and the inhibition state of ATPase activity. A hexameric ring model of EMCV 2C full length indicated that the C-terminal helix may get close to the N-terminal amphipathic helices to form a continuous positive region for RNA binding. The RNA-binding studies of EMCV 2C revealed that the RNA length is closely associated with the RNA-binding affinities and indicated that the substrate may wrap around the outer surface of the hexamer. Our studies provide a biochemical framework to guide the characterization of EMCV 2C and the essential role of arginine in cardioviral 2C functions.
Importance: Encephalomyocarditis virus (Cardiovirus A) is the causative agent of the homonymous disease, which may induce myocarditis, encephalitis, and reproductive disorders in various mammals. 2C protein is functionally indispensable and a promising target for drug development involving broad-spectrum picornaviral inhibitors. Here, an endogenous ATP molecule with a unique conformation was discovered by a combination of protein crystallography and high-performance liquid chromatography in the encephalomyocarditis virus (EMCV) 2C structure. Biochemical and structural characterization analysis of EMCV 2C revealed the critical role of conserved Arg311 in ATPase activity and self-oligomerization of EMCV 2C. The viral replication kinetics and infectivity study suggested that the residue negatively regulated the infectivity titer and virus encapsulation efficiency of EMCV and is, therefore, crucial for 2C protein to promote viral replication. Our systemic structure-function analysis provides unique insights into the function and regulation mechanism of cardioviral 2C protein.
Keywords: 2C; ATPase activity; crystal structure; encephalomyocarditis virus; endogenous ATP; molecular dynamic simulations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Zell R, Delwart E, Gorbalenya AE, Hovi T, King AMQ, Knowles NJ, Lindberg AM, Pallansch MA, Palmenberg AC, Reuter G, Simmonds P, Skern T, Stanway G, Yamashita T, Ictv Report Consortium . 2017. ICTV virus taxonomy profile: picornaviridae. J Gen Virol 98:2421–2422. doi: 10.1099/jgv.0.000911 - DOI - PMC - PubMed
-
- Reddacliff LA, Kirkland PD, Hartley WJ, Reece RL. 1997. Encephalomyocarditis virus infections in an Australian zoo. J Zoo Wildl Med 28:153–157. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

