Healthcare Utilization and Costs Among Patients with Acute Myeloid Leukemia Receiving Oral Azacitidine Maintenance Therapy Versus No Maintenance: A US Claims Database Study
- PMID: 39240504
- PMCID: PMC11480148
- DOI: 10.1007/s12325-024-02947-1
Healthcare Utilization and Costs Among Patients with Acute Myeloid Leukemia Receiving Oral Azacitidine Maintenance Therapy Versus No Maintenance: A US Claims Database Study
Abstract
Introduction: The substantial economic burden of acute myeloid leukemia (AML) could be reduced with post-remission maintenance therapies that delay relapse. Real-world healthcare resource utilization (HCRU) data and costs among patients with AML receiving oral azacitidine (Oral-AZA) maintenance therapy or no maintenance are not well understood. We characterize HCRU and costs among these patients in clinical practice in the USA.
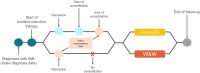
Methods: Data from IQVIA PharMetrics® Plus (January 1, 2016-June 30, 2022) were used. Patients ≥ 18 years who were newly diagnosed with AML, received first-line systemic induction therapy, and attained disease remission were eligible. Patients receiving Oral-AZA maintenance and those receiving no maintenance ("watch and wait" [W&W]) were matched 1:3 on baseline characteristics using propensity score matching (PSM) and followed until hematopoietic stem cell transplantation or end of continuous insurance enrollment, whichever occurred first. Outcomes included treatment patterns, inpatient and outpatient visits, and costs.
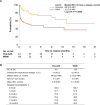
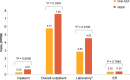
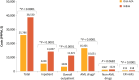
Results: After PSM, the Oral-AZA cohort included 43 patients and the W&W cohort 129. Of the 43 patients receiving Oral-AZA, 88.4% started at the recommended dose of 300 mg and 11.6% at 200 mg. The Oral-AZA cohort had significantly (p = 0.0025) longer median (95% CI) time to relapse from the index maintenance date (median not reached [NR; 9.0 months-NR] vs 3.3 months [0.8 months-NR]), and fewer per person per month (PPPM) hospitalizations (0.23 vs 0.61; p = 0.0005) and overall outpatient visits (5.77 vs 7.58; p = 0.0391) than the W&W cohort. Despite higher AML drug costs PPPM in the Oral-AZA cohort ($16,401 vs $10,651 for W&W), total healthcare costs PPPM were lower ($25,786 vs $38,530 for W&W; p < 0.0001).
Conclusions: Patients with newly diagnosed AML treated with Oral-AZA maintenance in clinical practice had prolonged remission and lower HCRU and costs than patients receiving no maintenance therapy. These findings underscore the clinical and economic value of Oral-AZA in clinical practice.
Keywords: Acute myeloid leukemia; Costs; Economic burden; Healthcare resource utilization; Maintenance therapy; Oral azacitidine; Real-world data; Remission.
© 2024. The Author(s).
Conflict of interest statement
Uma Borate has received grants/research support from and served as an advisory board member for AbbVie; has served as an advisory board member for Agios, Astellas, Blueprint Medicines, Genentech, Kura Oncology, Novartis, Servier, and Takeda; has received grants/research support from Incyte, Jazz Pharmaceuticals, and Pfizer; and has received honoraria from the RUNX1 Foundation. Karen Seiter has received research funding and consulting fees from Bristol Myers Squibb. Ravi Potluri and Debasish Mazumder declare employment with SmartAnalyst/Putnam Associates. Manoj Chevli, Thomas Prebet, Lona Gaugler, Maria Strocchia, and Jan Sieluk declare employment and stock ownership in Bristol Myers Squibb. Alberto Vasconcelos has received research funding support for travel/meeting attendance and declares employment and stock ownership in Bristol Myers Squibb.
Figures
Similar articles
-
Real-world treatment patterns and outcomes with oral azacitidine maintenance therapy in patients with acute myeloid leukemia.Cancer. 2025 Apr 15;131(8):e35845. doi: 10.1002/cncr.35845. Cancer. 2025. PMID: 40233158
-
Economic and Clinical Burden of Acute Myeloid Leukemia Episodes of Care in the United States: A Retrospective Analysis of a Commercial Payer Database.J Manag Care Spec Pharm. 2020 Jul;26(7):849-859. doi: 10.18553/jmcp.2020.19220. Epub 2020 Apr 13. J Manag Care Spec Pharm. 2020. PMID: 32281456 Free PMC article.
-
Azacitidine for the treatment of myelodysplastic syndrome, chronic myelomonocytic leukaemia and acute myeloid leukaemia.Health Technol Assess. 2010 May;14 Suppl 1:69-74. doi: 10.3310/hta14Suppl1/10. Health Technol Assess. 2010. PMID: 20507806 Review.
-
Management of adverse events in patients with acute myeloid leukemia in remission receiving oral azacitidine: experience from the phase 3 randomized QUAZAR AML-001 trial.J Hematol Oncol. 2021 Aug 28;14(1):133. doi: 10.1186/s13045-021-01142-x. J Hematol Oncol. 2021. PMID: 34454540 Free PMC article. Clinical Trial.
-
Azacitidine for Treating Acute Myeloid Leukaemia with More Than 30 % Bone Marrow Blasts: An Evidence Review Group Perspective of a National Institute for Health and Care Excellence Single Technology Appraisal.Pharmacoeconomics. 2017 Mar;35(3):363-373. doi: 10.1007/s40273-016-0453-5. Pharmacoeconomics. 2017. PMID: 27752999 Review.
References
-
- Shimony S, Stahl M, Stone RM. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(3):502–26. - PubMed
-
- National Cancer Institute. SEER Cancer Stat Facts: Leukemia — Acute Myeloid Leukemia. https://seer.cancer.gov/statfacts/html/amyl.html. Accessed 6 Mar 2024.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

