Long-Term Safety and Efficacy Evaluation of Travoprost Intracameral Implant Based on Pooled Analyses from Two Phase III Trials
- PMID: 39240530
- PMCID: PMC11512891
- DOI: 10.1007/s40265-024-02074-9
Long-Term Safety and Efficacy Evaluation of Travoprost Intracameral Implant Based on Pooled Analyses from Two Phase III Trials
Abstract
Aim: The purpose of this study was to conduct and interpret a pooled 12-month analysis of two prospective, multi-center, randomized, double-masked, controlled trials designed to assess the efficacy and safety of the travoprost intracameral implant (slow-eluting [SE] implant in development as a new therapeutic and fast-eluting [FE] implant included for masking purposes) in subjects with open-angle glaucoma (OAG) or ocular hypertension (OHT).
Methods: Subjects with OAG or OHT, on 0-3 intraocular pressure (IOP)-lowering medications, baseline unmedicated mean diurnal IOP of ≥ 21 mmHg, and IOP ≤ 36 mmHg at each baseline diurnal timepoint, received either a travoprost implant and twice-daily (BID) placebo eye drops or BID timolol 0.5% eye drops and a sham procedure. Subjects were followed through 12 months and assessed for IOP, reduction in topical IOP-lowering medications, and safety parameters including treatment-emergent adverse events (TEAEs). IOP at 8AM was prospectively collected at all study visits through 12 months and diurnal IOP, measured at 8AM, 10AM, and 4PM, was prospectively collected at baseline, day 10, week 6, and months 3 and 12.
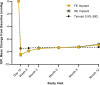
Results: A total of 1150 subjects were randomized (385 FE implant, 380 SE implant, and 385 sham/timolol) across the two trials. Statistical non-inferiority to timolol and clinically relevant reductions in 8AM IOPs were demonstrated at month 12. In more detail, both implant groups demonstrated statistical non-inferiority to timolol and clinically relevant reductions from baseline in mean diurnal IOP at all visits over the 12-month evaluation period when diurnal IOP was collected. Additionally, both implant groups demonstrated robust treatment effect based on 8AM average IOP from day 10 through the specified visit which ranged from day 10 to month 12 from 6.9 to 8.5 mmHg in the FE implant group; 6.8 to 8.5 mmHg in the SE implant group; and 7.3 to 7.5 mmHg in the sham/timolol group. With regards to reduction in topical pharmacotherapy, at month 12, 77.6% of FE and 81.4% of SE implant eyes were completely free of all topical IOP-lowering medications and a significantly greater proportion of FE and SE implant eyes (89.9% and 93.0%) versus sham/timolol eyes (66.9%) were on the same or fewer topical IOP-lowering medications compared with pre-study (p < 0.0001). Furthermore, of subjects on topical IOP medications at screening, a significantly greater proportion of FE implant (80.2%) and SE implant (85.1%) eyes versus sham/timolol (22.8%) eyes were on fewer topical IOP-lowering medications at month 12 compared with pre-study (p < 0.0001). Lastly, of SE implant eyes on same or fewer topical IOP-lowering medications at month 12, the average through month 12 decreased by 0.9 medications, and of those SE implant eyes on fewer topical IOP-lowering medications compared with pre-study, the average through month 12 decreased by 1.4 medications. The most common TEAEs related to study treatment were hyperemia (conjunctival or ocular), iritis, and IOP increased.
Conclusion: The travoprost intracameral implant demonstrated robust IOP-lowering efficacy that was sustained and statistically non-inferior to timolol over the entire 12 months, resulting in a significant reduction in topical IOP-lowering medication use, with the majority of SE implant eyes remaining completely free of all topical IOP-lowering medications. In addition, the implant demonstrated a favorable safety and tolerability profile based on this pooled 12-month analysis of two pivotal trials.
Trial registration: ClinicalTrials.gov identifiers NCT03519386 (registered May 09, 2018) and NCT03868124 (registered March 08, 2019).
© 2024. The Author(s).
Conflict of interest statement
I. Paul Singh has received consulting fees from Allergan, Alcon, Bausch + Lomb, Glaukos, iStar Medical, New World Medical, Nova Eye, and Sight Sciences; and lecture fees from Allergan, Alcon, Bausch + Lomb, Glaukos, iStar Medical, New World Medical, Nova Eye, Sight Sciences, Thea and Viatris. John P. Berdahl has received consulting fees, has been a paid advisory board member or received fees for attending a meeting for AbbVie, Aerpio, Alcon, Aldeyra, Aurea Medical, Aurion Biotech/CorneaGen, Balance Ophthalmics, Bausch and Lomb, Dakota Lions Eye Bank, Elios Vision Inc., Equinox, Expert Opinion, Glaukos, Gore, Imprimis, Interfeen, iRenix, Iacta Pharmaceuticals, IVERIC bio, Inc., JNJ, Kala, Kedalion, MELT Pharmaceuticals, MicroOptx, New World Medical, Ocular Surgical Data, Ocular Therapeutix, Omega Ophthalmic, Orasis, Oyster Point, RxSight, Santen, Sight Sciences, Surface Inc., Tarsus, Tear Clear, Vertex Ventures, ViaLase, Vittamed, Vance Thompson Vision, Versea Biologics, Visionary Ventures, Visus and Zeiss; has received lecture fees (honoraria), travel fees or reimbursements when speaking for AbbVie, Alcon and Glaukos; has equity ownership/stock options of Aurion Biotech/CorneaGen, Balance Ophthalmics, Equinox, Expert Opinion, Interfeen, Ocular Surgical Data, Omega Ophthalmic, Surface Inc, Vance Thompson Vision, Verana Health and Zeiss; owns stock of Glaukos; has patents and/or royalties with Imprimis. Steven R. Sarkisian Jr is a consultant/advisor for Alcon, Allergan, Bausch + Lomb, Beaver-Visitec International, Glaukos, Icare USA, Katena Products, MicroSurgical Technology, Ocular Science, Santen, and Sight Sciences; is on the speaker’s bureau for Alcon, Allergan, and Bausch + Lomb; has received grant support from Alcon, Allergan, Allysta Pharmaceuticals, Elios, Glaukos, iSTAR Medical, Ocular Science, Ocular Therapeutix, and Sight Sciences; and holds stock or stock options in Ocular Science and Sight Sciences. Lilit A. Voskanyan has received research support from Glaukos. Robert E. Ang has been a speaker for and has received research support from Glaukos. Long V. Doan, David Applegate, Yannan Shen, L. Jay Katz, Angela C. Kothe, and Tomas Navratil are employees of Glaukos Corporation and may have Glaukos stock and/or stock options.
Figures

References
-
- Gedde SJ, Vinod K, Wright MM, American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Panel, et al. Primary open-angle glaucoma preferred practice pattern®. Ophthalmology. 2021;128(1):71–150.
-
- Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140:598–606. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

