Reduced Nicotine Cigarettes and E-Cigarettes in High-Risk Populations: 3 Randomized Clinical Trials
- PMID: 39240566
- PMCID: PMC11380105
- DOI: 10.1001/jamanetworkopen.2024.31731
Reduced Nicotine Cigarettes and E-Cigarettes in High-Risk Populations: 3 Randomized Clinical Trials
Abstract
Importance: Prohibiting the sale of commonly preferred e-cigarette flavors (eg, fruity and sweet) to discourage use among youths poses a risk of diminishing efforts to decrease smoking in adults.
Objective: To compare reductions in smoking achieved in adults with psychiatric conditions or lower educational level using very low nicotine content (VLNC) cigarettes alone, combined with e-cigarettes limited to tobacco flavor (TF), or combined with e-cigarettes in participant-preferred flavors.
Design, setting, and participants: Three randomized clinical trials were conducted for 16 weeks from October 2020 through November 2023 at the University of Vermont, Brown University, and Johns Hopkins University. Participants were adults who smoked daily and were not planning to quit in the next 30 days. These participants were from 3 at-risk populations: those with affective disorders, exemplifying mental illness; those with opioid use disorder, exemplifying substance use disorders; and females of reproductive age with a high-school education or less, exemplifying lower educational level. Participants were randomly assigned to 1 of 4 experimental conditions: (1) normal nicotine content (NNC) cigarettes only; (2) VLNC cigarettes only; (3) VLNC cigarettes plus e-cigarettes with classic TF (hereafter, VLNC + TF); and (4) VLNC cigarettes plus e-cigarettes with preferred flavors (hereafter, VLNC + PF).
Interventions: The NNC cigarettes contained 15.8 mg nicotine/g tobacco, the VLNC cigarettes contained 0.4 mg nicotine/g tobacco, the VLNC + TF had pods containing 5% nicotine by weight and only classic TF, and the VLNC + PF had pods containing 5% nicotine in 8 flavors (including fruity and sweet) from which participants selected 3 flavors.
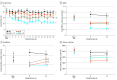
Main outcomes and measures: The primary outcome was mean total cigarettes smoked per day (CPD) during week 16. Tobacco-related biomarkers were assessed, including total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), a tobacco-specific carcinogen.
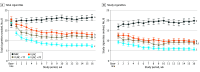
Results: A total of 326 participants (mean [SD] age, 40.09 [10.79] years; 243 females [74.5%]) from 3 randomized clinical trials were included. The VLNC cigarettes decreased total CPD, with least square (LS) means (SEMs) of 22.54 (1.59) in the NNC, 14.32 (1.32) in the VLNC, 11.76 (1.18) in the VLNC + TF, and 7.63 (0.90) in the VLNC + PF conditions. Each VLNC condition differed significantly from NNC, with an adjusted mean difference (AMD) of -8.21 (95% CI, -12.27 to -4.16; P < .001) in the VLNC, -10.78 (95% CI, -14.67 to -6.90; P < .001) in the VLNC + TF, and -14.91 (95% CI, -18.49 to -11.33; P < .001) in the VLNC + PF conditions. Participants in the VLNC + PF condition also decreased smoking below the VLNC and the VLNC + TF conditions (AMDs, -6.70 [95% CI, -9.84 to -3.55; P < .001] and -4.13 [95% CI, -7.05 to -1.21; P = .02]); the VLNC and VLNC + TF conditions did not differ significantly. Consistent with decreases in CPD, NNAL levels in the VLNC + PF condition were lower than in all other conditions, with AMDs (in pmol/mg creatinine) of -0.94 (95% CI, -1.41 to -0.47; P < .001) compared with the NNC condition, -0.47 (95% CI, -0.87 to -0.08; P = .03) compared with the VLNC condition, and -0.46 (95% CI, -0.83 to -0.10; P = .04) compared with the VLNC + TF condition.
Conclusions and relevance: These results provide further evidence that a reduced-nicotine standard for cigarettes has the potential to decrease smoking and tobacco-toxicant exposure in high-risk populations and that these effects may be enhanced when adults can access e-cigarettes in commonly preferred flavors.
Trial registration: ClinicalTrials.gov Identifiers: NCT04092387, NCT04090879, NCT04092101.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention . Health effects of cigarette smoking. Accessed November 24, 2023. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/e...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

