Sugar signaling modulates SHOOT MERISTEMLESS expression and meristem function in Arabidopsis
- PMID: 39240964
- PMCID: PMC11406306
- DOI: 10.1073/pnas.2408699121
Sugar signaling modulates SHOOT MERISTEMLESS expression and meristem function in Arabidopsis
Abstract
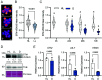
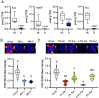
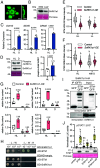
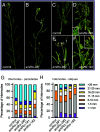
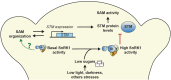
In plants, development of all above-ground tissues relies on the shoot apical meristem (SAM) which balances cell proliferation and differentiation to allow life-long growth. To maximize fitness and survival, meristem activity is adjusted to the prevailing conditions through a poorly understood integration of developmental signals with environmental and nutritional information. Here, we show that sugar signals influence SAM function by altering the protein levels of SHOOT MERISTEMLESS (STM), a key regulator of meristem maintenance. STM is less abundant in inflorescence meristems with lower sugar content, resulting from plants being grown or treated under limiting light conditions. Additionally, sucrose but not light is sufficient to sustain STM accumulation in excised inflorescences. Plants overexpressing the α1-subunit of SUCROSE-NON-FERMENTING1-RELATED KINASE 1 (SnRK1) accumulate less STM protein under optimal light conditions, despite higher sugar accumulation in the meristem. Furthermore, SnRK1α1 interacts physically with STM and inhibits its activity in reporter assays, suggesting that SnRK1 represses STM protein function. Contrasting the absence of growth defects in SnRK1α1 overexpressors, silencing SnRK1α in the SAM leads to meristem dysfunction and severe developmental phenotypes. This is accompanied by reduced STM transcript levels, suggesting indirect effects on STM. Altogether, we demonstrate that sugars promote STM accumulation and that the SnRK1 sugar sensor plays a dual role in the SAM, limiting STM function under unfavorable conditions but being required for overall meristem organization and integrity under favorable conditions. This highlights the importance of sugars and SnRK1 signaling for the proper coordination of meristem activities.
Keywords: Arabidopsis thaliana; plant development; shoot apical meristem; sugar signaling.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Barton M. K., Twenty years on: The inner workings of the shoot apical meristem, a developmental dynamo. Dev. Biol. 341, 95–113 (2010). - PubMed
-
- Galli M., Gallavotti A., Expanding the regulatory network for meristem size in plants. Trends Genet. 32, 372–383 (2016). - PubMed
-
- Lopes F. L., Galvan-Ampudia C., Landrein B., WUSCHEL in the shoot apical meristem: Old player, new tricks. J. Exp. Bot. 72, 1527–1535 (2021). - PubMed
-
- Long J. A., Moan E. I., Medford J. I., Barton M. K., A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379, 66–69 (1996). - PubMed
-
- Barton M. K., Poethig R. S., Formation of the shoot apical meristem in Arabidopsis thaliana: An analysis of development in the wild type and in the shoot meristemless mutant. Development 119, 823–831 (1993).
MeSH terms
Substances
Grants and funding
- UIDB/04551/2020/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- UIDP/04551/2020/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- LA/P/0087/2020/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- PTDC/BIA-FBT/4942/2020/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- PTDC/BIA-BID/32347/2017/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- Associated Laboratory) PTDC/BIA-FBT/4942/2020 PTDC/BIA-BID/32347/2017 PD/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- n/a/Max Planck Society
- GAT3395-PR4B/Gatsby Charitable Foundation (GATSBY)
- CF392/EPA Cephalosporin Fund (E P A Cephalosporin Fund)
- 0013575/| John Fell Fund, University of Oxford (John Fell OUP Research Fund)
- 2022.08339.PTDC/MEC | Fundação para a Ciência e a Tecnologia (FCT)
LinkOut - more resources
Full Text Sources
Medical

