Cost-effectiveness of vaccinating adults aged 60 years and older against respiratory syncytial virus
- PMID: 39241353
- PMCID: PMC11848646
- DOI: 10.1016/j.vaccine.2024.126294
Cost-effectiveness of vaccinating adults aged 60 years and older against respiratory syncytial virus
Abstract
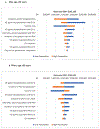
Respiratory syncytial virus (RSV) causes substantial morbidity and mortality in older adults. In May 2023, two subunit RSV vaccines (Arexvy [GSK] and Abrysvo [Pfizer]) received approval from the U.S. Food and Drug Administration (FDA). In June 2023, ACIP recommended that adults aged ≥60 years may receive a single dose of RSV vaccine, using shared clinical decision-making. In support of development of this policy, our objective was to assess the cost-effectiveness of RSV vaccination in the general population in this age group. We used a decision-analytical model of RSV over a two-year timeframe using data from published literature, FDA documents, epidemiological databases, and manufacturer data. We tracked RSV-associated outpatient, emergency department, inpatient healthcare utilization, RSV-attributable deaths, quality-adjusted life-years lost (QALYs), and societal costs. The societal cost per QALY saved from RSV vaccination depended on age group and product: adults aged ≥60 years, $196,842 for GSK's vaccine and $176,557 for Pfizer's vaccine; adults ≥65 years, $162,138 for GSK and $146,543 for Pfizer; adults 60- <65 years, $385,829 for GSK and $331,486 for Pfizer. Vaccine efficacy, incidence of RSV hospitalization, and vaccine cost had the greatest influence on cost per QALY. Cost per QALY saved decreased as the age of those vaccinated increased. Inputs such as long-term efficacy are uncertain. RSV vaccination in adults aged ≥60 years may be cost-effective, particularly in those of more advanced age. Lower vaccine acquisition costs and persistent efficacy beyond two RSV seasons would render RSV vaccination more cost-effective for a broader target population. PRIMARY FUNDING SOURCE: US Centers for Disease Control and Prevention.
Keywords: Adult RSV disease; Cost-effectiveness; Vaccination.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: David W. Hutton, Lisa A. Prosser, Angela M. Rose, and Kerra Mercon report financial support was provided by Centers for Disease Control and Prevention. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of mortality attributable to influenza and RSV in the United States during 1997-2009 by influenza type or subtype, age, cause of death, and risk status. Influenza Other Respir Viruses 2014;8(5):507–15. 10.1111/irv.12258. - DOI - PMC - PubMed
-
- Melgar MB, Amadea. Evidence to Recommendations Framework: Respiratory Syncytial Virus (RSV) in Adults Pfizer bivalent RSVpreF vaccine in older adults GSK adjuvanted RSVPreF3 vaccine in older adults. Accessed September 27, https://stacks.cdc.gov/view/cdc/129996; 2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

