Detecting altered hepatic lipid oxidation by MRI in an animal model of MASLD
- PMID: 39241774
- PMCID: PMC11525016
- DOI: 10.1016/j.xcrm.2024.101714
Detecting altered hepatic lipid oxidation by MRI in an animal model of MASLD
Abstract
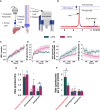
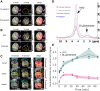
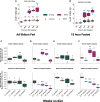
Metabolic dysfunction-associated steatotic liver disease (MASLD) prevalence is increasing annually and affects over a third of US adults. MASLD can progress to metabolic dysfunction-associated steatohepatitis (MASH), characterized by severe hepatocyte injury, inflammation, and eventual advanced fibrosis or cirrhosis. MASH is predicted to become the primary cause of liver transplant by 2030. Although the etiology of MASLD/MASH is incompletely understood, dysregulated fatty acid oxidation is implicated in disease pathogenesis. Here, we develop a method for estimating hepatic β-oxidation from the metabolism of [D15]octanoate to deuterated water and detection with deuterium magnetic resonance methods. Perfused livers from a mouse model of MASLD reveal dysregulated hepatic β-oxidation, findings that corroborate in vivo imaging. The high-fat-diet-induced MASLD mouse studies indicate that decreased β-oxidative efficiency in the fatty liver could serve as an indicator of MASLD progression. Furthermore, our method provides a clinically translatable imaging approach for determining hepatic β-oxidation efficiency.
Keywords: MASLD; X-nucleus imaging; deuterium MRI; metabolic flux; octanoate; steatosis; β-oxidation.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.E.M. and R.M. are holders of patent ##T17984US002 related to metabolic imaging of HDO.
Figures

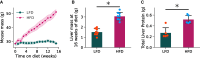



References
-
- Cusi K., Isaacs S., Barb D., Basu R., Caprio S., Garvey W.T., Kashyap S., Mechanick J.I., Mouzaki M., Nadolsky K., et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings. Endocr. Pract. 2022;28:528–562. doi: 10.1016/j.eprac.2022.03.010. - DOI - PubMed
-
- Rinella M.E., Neuschwander-Tetri B.A., Siddiqui M.S., Abdelmalek M.F., Caldwell S., Barb D., Kleiner D.E., Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797–1835. doi: 10.1097/HEP.0000000000000323. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

