Hearing restoration by gene replacement therapy for a multisite-expressed gene in a mouse model of human DFNB111 deafness
- PMID: 39241775
- PMCID: PMC11480802
- DOI: 10.1016/j.ajhg.2024.08.008
Hearing restoration by gene replacement therapy for a multisite-expressed gene in a mouse model of human DFNB111 deafness
Erratum in
-
Hearing restoration by gene replacement therapy for a multisite-expressed gene in a mouse model of human DFNB111 deafness.Am J Hum Genet. 2025 Sep 4;112(9):2249. doi: 10.1016/j.ajhg.2025.08.011. Epub 2025 Aug 20. Am J Hum Genet. 2025. PMID: 40840449 Free PMC article. No abstract available.
Abstract
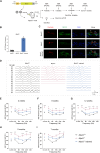
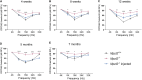
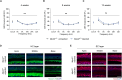
Gene therapy has made significant progress in the treatment of hereditary hearing loss. However, most research has focused on deafness-related genes that are primarily expressed in hair cells with less attention given to multisite-expressed deafness genes. MPZL2, the second leading cause of mild-to-moderate hereditary deafness, is widely expressed in different inner ear cells. We generated a mouse model with a deletion in the Mpzl2 gene, which displayed moderate and slowly progressive hearing loss, mimicking the phenotype of individuals with DFNB111. We developed a gene replacement therapy system mediated by AAV-ie for efficient transduction in various types of cochlear cells. AAV-ie-Mpzl2 administration significantly lowered the auditory brainstem response and distortion product otoacoustic emission thresholds of Mpzl2-/- mice for at least seven months. AAV-ie-Mpzl2 delivery restored the structural integrity in both outer hair cells and Deiters cells. This study suggests the potential of gene therapy for MPZL2-related deafness and provides a proof of concept for gene therapy targeting other deafness-related genes that are expressed in different cell populations in the cochlea.
Keywords: DFNB111; MPZL2; gene therapy; hereditary hearing loss.
Copyright © 2024 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Alford R.L., Arnos K.S., Fox M., Lin J.W., Palmer C.G., Pandya A., Rehm H.L., Robin N.H., Scott D.A., Yoshinaga-Itano C., et al. American College of Medical Genetics and Genomics guideline for the clinical evaluation and etiologic diagnosis of hearing loss. Genet. Med. 2014;16:347–355. doi: 10.1038/gim.2014.2. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

