Effectiveness of Disease-Modifying Treatment on Spinal Cord Lesion Formation in Relapse-Onset Multiple Sclerosis: An MSBase Registry Study
- PMID: 39242483
- PMCID: PMC11486785
- DOI: 10.1007/s40263-024-01115-x
Effectiveness of Disease-Modifying Treatment on Spinal Cord Lesion Formation in Relapse-Onset Multiple Sclerosis: An MSBase Registry Study
Abstract
Background: Spinal cord lesions in multiple sclerosis (MS) have considerable impact on disability. High-efficacy disease-modifying treatments (hDMTs) are associated with greater reduction of relapses and new brain lesions compared to low-efficacy treatments (lDMTs). Knowledge on the impact of DMTs on cord lesion formation is limited as these outcome measures were not included in MS treatment trials. This study aims to investigate whether hDMTs reduce the formation of cord lesions more effectively than lDMTs.
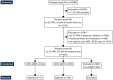
Methods: Patients with relapse-onset MS, a cord magnetic resonance imaging (MRI) within 6 months before/after initiation of their first DMT and ≥1 cord MRI at follow-up (interval > 6 months) were extracted from the MSBase registry (ACTRN12605000455662). Patients treated with hDMTs ≥90% or lDMTs ≥90% of follow-up duration were considered the hDMT and lDMT groups, respectively. Matching was performed using propensity scores. Cox proportional hazards models were used to estimate the hazards of new cord lesions, brain lesions and relapses.
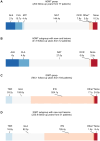
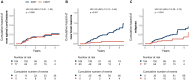
Results: Ninety-four and 783 satisfied hDMT and lDMT group criteria, respectively. Seventy-seven hDMT patients were matched to 184 lDMT patients. In the hDMT group there was no evidence of reduction of new cord lesions (hazard ratio [HR] 0.99 [95% CI 0.51, 1.92], p = 0.97), while there were fewer new brain lesions (HR 0.22 [95% CI 0.10, 0.49], p < 0.001) and fewer relapses (HR 0.45 [95% CI 0.28, 0.72], p = 0.004).
Conclusion: A potential discrepancy exists in the effect of hDMTs over lDMTs in preventing spinal cord lesions versus brain lesions and relapses. While hDMTs provided a significant reduction for the latter when compared to lDMTs, there was no significant reduction in new spinal cord lesions.
© 2024. The Author(s).
Conflict of interest statement
Serkan Ozakbas has nothing to disclose. Daniel Kreiter and Oliver Gerlach received institutional research grants. Tomas Kalincik served on scientific advisory boards for MS International Federation and World Health Organization, BMS, Roche, Janssen, Sanofi Genzyme, Novartis, Merck and Biogen, steering committee for Brain Atrophy Initiative by Sanofi Genzyme, received conference travel support and/or speaker honoraria from WebMD Global, Eisai, Novartis, Biogen, Roche, Sanofi-Genzyme, Teva, BioCSL and Merck and received research or educational event support from Biogen, Novartis, Genzyme, Roche, Celgene and Merck. Tomas Kalincik is also an Editorial Board member of CNS Drugs. He was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Raymond Hupperts received institutional research grants and fees for lectures and advisory boards from Biogen, Merck and Genzyme-Sanofi. Francesco Patti received personal compensation for serving on advisory board for Almirall, Alexion, Biogen, Bristol, Berck, Novartis and Roche. He further received research grants by Biogen, Merck and Roche and by FISM, Reload Association (Onlus), Italian Health Minister, University of Catania. Daniele Spitaleri received honoraria as a consultant on scientific advisory boards by Bayer-Schering, Novartis and Sanofi-Aventis and compensation for travel from Novartis, Biogen, Sanofi Aventis, Teva and Merck. Matteo Foschi received travel and meeting attendance support from Novartis, Biogen, Roche, Sanofi-Genzyme and Merck. Davide Maimone received speaker honoraria for Advisory Board and travel grants from Almirall, Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva. Bassem Yamout received honoraria as a speaker and member of scientific advisory boards from Sanofi, Bayer, Biogen, Merck, Janssen, Novartis, Roche and Aspen. Samia J. Khoury received compensation for scientific advisory board activity from Merck and Roche and received compensation for serving on the IDMC for Biogen. Jeannette Lechner-Scott travel compensation from Novartis, Biogen, Roche and Merck. Her institution receives the honoraria for talks and advisory board commitment as well as research grants from Biogen, Merck, Roche, TEVA and Novartis.
Figures

References
-
- Bot JC, Barkhof F, Polman CH, Lycklama a Nijeholt GJ, de Groot V, Bergers E, et al. Spinal cord abnormalities in recently diagnosed MS patients: added value of spinal MRI examination. Neurology. 2004;62:226–33. - PubMed
-
- Lukas C, Sombekke MH, Bellenberg B, Hahn HK, Popescu V, Bendfeldt K, et al. Relevance of spinal cord abnormalities to clinical disability in multiple sclerosis: MR imaging findings in a large cohort of patients. Radiology. 2013;269:542–52. - PubMed
-
- Brownlee WJ, Altmann DR, Prados F, Miszkiel KA, Eshaghi A, Gandini Wheeler-Kingshott CAM, et al. Early imaging predictors of long-term outcomes in relapse-onset multiple sclerosis. Brain. 2019;142:2276–87. - PubMed
-
- Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol. 2019;15:287–300. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

