Orphan receptor-GPR52 inverse agonist efficacy in ameliorating chronic stress-related deficits in reward motivation and phasic accumbal dopamine activity in mice
- PMID: 39242529
- PMCID: PMC11379876
- DOI: 10.1038/s41398-024-03081-w
Orphan receptor-GPR52 inverse agonist efficacy in ameliorating chronic stress-related deficits in reward motivation and phasic accumbal dopamine activity in mice
Abstract
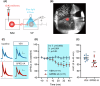
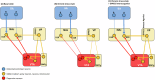
Reward processing dysfunctions e.g., anhedonia, apathy, are common in stress-related neuropsychiatric disorders including depression and schizophrenia, and there are currently no established therapies. One potential therapeutic approach is restoration of reward anticipation during appetitive behavior, deficits in which co-occur with attenuated nucleus accumbens (NAc) activity, possibly due to NAc inhibition of mesolimbic dopamine (DA) signaling. Targeting NAc regulation of ventral tegmental area (VTA) DA neuron responsiveness to reward cues could involve either the direct or indirect-via ventral pallidium (VP)-pathways. One candidate is the orphan G protein-coupled receptor GPR52, expressed by DA receptor 2 NAc neurons that project to VP. In mouse brain-slice preparations, GPR52 inverse agonist (GPR52-IA) attenuated evoked inhibitory postsynaptic currents at NAc-VP neurons, which could disinhibit VTA DA neurons. A mouse model in which chronic social stress leads to reduced reward learning and effortful motivation was applied to investigate GPR52-IA behavioral effects. Control and chronically stressed mice underwent a discriminative learning test of tone-appetitive behavior-sucrose reinforcement: stress reduced appetitive responding and discriminative learning, and these anticipatory behaviors were dose-dependently reinstated by GPR52-IA. The same mice then underwent an effortful motivation test of operant behavior-tone-sucrose reinforcement: stress reduced effortful motivation and GPR52-IA dose-dependently restored it. In a new cohort, GRABDA-sensor fibre photometry was used to measure NAc DA activity during the motivation test: in stressed mice, reduced motivation co-occurred with attenuated NAc DA activity specifically to the tone that signaled reinforcement of effortful behavior, and GPR52-IA ameliorated both deficits. These findings: (1) Demonstrate preclinical efficacy of GPR52 inverse agonism for stress-related deficits in reward anticipation during appetitive behavior. (2) Suggest that GPR52-dependent disinhibition of the NAc-VP-VTA-NAc circuit, leading to increased phasic NAc DA signaling of earned incentive stimuli, could account for these clinically relevant effects.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

