Trypanosoma cruzi reprograms mitochondrial metabolism within the anterior midgut of its vector Rhodnius prolixus during the early stages of infection
- PMID: 39242536
- PMCID: PMC11380418
- DOI: 10.1186/s13071-024-06415-1
Trypanosoma cruzi reprograms mitochondrial metabolism within the anterior midgut of its vector Rhodnius prolixus during the early stages of infection
Abstract
Background: Trypanosoma cruzi is transmitted to humans by hematophagous bugs belonging to the Triatominae subfamily. Its intra-vectorial cycle is complex and occurs exclusively in the insect's midgut. Dissecting the elements involved in the cross-talk between the parasite and its vector within the digestive tract should provide novel targets for interrupting the parasitic life cycle and affecting vectorial competence. These interactions are shaped by the strategies that parasites use to infect and exploit their hosts, and the host's responses that are designed to detect and eliminate parasites. The objective of the current study is to characterize the impact of T. cruzi establishment within its vector on the dynamics of its midgut.
Methods: In this study, we evaluated the impact of T. cruzi infection on protein expression within the anterior midgut of the model insect Rhodnius prolixus at 6 and 24 h post-infection (hpi) using high-throughput quantitative proteomics.
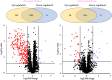
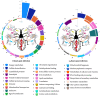
Results: Shortly after its ingestion, the parasite modulates the proteome of the digestive epithelium by upregulating 218 proteins and negatively affecting the expression of 11 proteins involved in a wide array of cellular functions, many of which are pivotal due to their instrumental roles in cellular metabolism and homeostasis. This swift response underscores the intricate manipulation of the vector's cellular machinery by the parasite. Moreover, a more in-depth analysis of proteins immediately induced by the parasite reveals a pronounced predominance of mitochondrial proteins, thereby altering the sub-proteomic landscape of this organelle. This includes various complexes of the respiratory chain involved in ATP generation. In addition to mitochondrial metabolic dysregulation, a significant number of detoxifying proteins, such as antioxidant enzymes and P450 cytochromes, were immediately induced by the parasite, highlighting a stress response.
Conclusions: This study is the first to illustrate the response of the digestive epithelium upon contact with T. cruzi, as well as the alteration of mitochondrial sub-proteome by the parasite. This manipulation of the vector's physiology is attributable to the cascade activation of a signaling pathway by the parasite. Understanding the elements of this response, as well as its triggers, could be the foundation for innovative strategies to control the transmission of American trypanosomiasis, such as the development of targeted interventions aimed at disrupting parasite proliferation and transmission within the triatomine vector.
Keywords: Chagas disease; Host–parasite interaction; Mitochondria; Quantitative proteomics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Pan American Health Organization. Chagas disease. Available online : https://www.paho.org/en/topics/chagas-disease. 2022. Accessed 1 Dec 2023.
-
- Chagas C. Nova tripanozomíase humana. Estudos sobre a morfologia e ciclo evolutivo do Schizotrypanum cruzi n gen., n. sp. agente etiológico de nova entidade mórbida do homem. Mem Inst Oswaldo Cruz. 1909;1:159–218. 10.1590/S0074-02761909000200008 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

