Osteopontin deficiency promotes cartilaginous endplate degeneration by enhancing the NF-κB signaling to recruit macrophages and activate the NLRP3 inflammasome
- PMID: 39242551
- PMCID: PMC11379908
- DOI: 10.1038/s41413-024-00355-3
Osteopontin deficiency promotes cartilaginous endplate degeneration by enhancing the NF-κB signaling to recruit macrophages and activate the NLRP3 inflammasome
Abstract
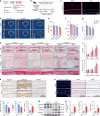
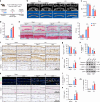
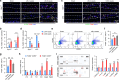
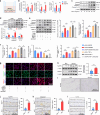
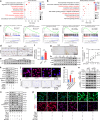
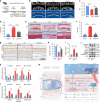
Intervertebral disc degeneration (IDD) is a major cause of discogenic pain, and is attributed to the dysfunction of nucleus pulposus, annulus fibrosus, and cartilaginous endplate (CEP). Osteopontin (OPN), a glycoprotein, is highly expressed in the CEP. However, little is known on how OPN regulates CEP homeostasis and degeneration, contributing to the pathogenesis of IDD. Here, we investigate the roles of OPN in CEP degeneration in a mouse IDD model induced by lumbar spine instability and its impact on the degeneration of endplate chondrocytes (EPCs) under pathological conditions. OPN is mainly expressed in the CEP and decreases with degeneration in mice and human patients with severe IDD. Conditional Spp1 knockout in EPCs of adult mice enhances age-related CEP degeneration and accelerates CEP remodeling during IDD. Mechanistically, OPN deficiency increases CCL2 and CCL5 production in EPCs to recruit macrophages and enhances the activation of NLRP3 inflammasome and NF-κB signaling by facilitating assembly of IRAK1-TRAF6 complex, deteriorating CEP degeneration in a spatiotemporal pattern. More importantly, pharmacological inhibition of the NF-κB/NLRP3 axis attenuates CEP degeneration in OPN-deficient IDD mice. Overall, this study highlights the importance of OPN in maintaining CEP and disc homeostasis, and proposes a promising therapeutic strategy for IDD by targeting the NF-κB/NLRP3 axis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

