RAB17 promotes endometrial cancer progression by inhibiting TFRC-dependent ferroptosis
- PMID: 39242574
- PMCID: PMC11379720
- DOI: 10.1038/s41419-024-07013-w
RAB17 promotes endometrial cancer progression by inhibiting TFRC-dependent ferroptosis
Abstract
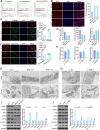
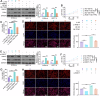
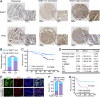
Studies have indicated that RAB17 expression levels are associated with tumor malignancy, and RAB17 is more highly expressed in endometrial cancer (EC) tissues than in peritumoral tissues. However, the roles and potential mechanisms of RAB17 in EC remain undefined. The present study confirmed that the expression of RAB17 facilitates EC progression by suppressing cellular ferroptosis-like alterations. Mechanistically, RAB17 attenuated ferroptosis in EC cells by inhibiting transferrin receptor (TFRC) protein expression in a ubiquitin proteasome-dependent manner. Because EC is a blood-deprived tumor with a poor energy supply, the relationship between RAB17 and hypoglycemia was investigated. RAB17 expression was increased in EC cells incubated in low-glucose medium. Moreover, low-glucose medium limited EC cell ferroptosis and promoted EC progression through the RAB17-TFRC axis. The in vitro results were corroborated by in vivo studies and clinical data. Overall, the present study revealed that increased RAB17 promotes the survival of EC cells during glucose deprivation by inhibiting the onset of TFRC-dependent ferroptosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

