Maintenance of magnesium homeostasis by NUF2 promotes protein synthesis and anaplastic thyroid cancer progression
- PMID: 39242581
- PMCID: PMC11379715
- DOI: 10.1038/s41419-024-07041-6
Maintenance of magnesium homeostasis by NUF2 promotes protein synthesis and anaplastic thyroid cancer progression
Erratum in
-
Correction: Maintenance of magnesium homeostasis by NUF2 promotes protein synthesis and anaplastic thyroid cancer progression.Cell Death Dis. 2024 Nov 18;15(11):842. doi: 10.1038/s41419-024-07174-8. Cell Death Dis. 2024. PMID: 39557824 Free PMC article. No abstract available.
-
Correction: Maintenance of magnesium homeostasis by NUF2 promotes protein synthesis and anaplastic thyroid cancer progression.Cell Death Dis. 2025 Mar 11;16(1):166. doi: 10.1038/s41419-025-07348-y. Cell Death Dis. 2025. PMID: 40069170 Free PMC article. No abstract available.
Abstract
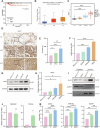
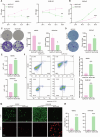
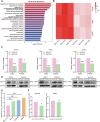
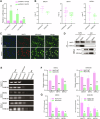
Thyroid cancer is the most frequently observed endocrine-related malignancy among which anaplastic thyroid cancer (ATC) is the most fatal subtype. The synthesis of protein is active to satisfy the rapid growth of ATC tumor, but the mechanisms regulating protein synthesis are still unknown. Our research revealed that kinetochore protein NUF2 played an essential role in protein synthesis and drove the progression of ATC. The prognosis of patients with thyroid carcinoma was positively correlated with high NUF2 expression. Depletion of NUF2 in ATC cells notably inhibited the proliferation and induced apoptosis, while overexpression of NUF2 facilitated ATC cell viability and colony formation. Deletion of NUF2 significantly suppressed the growth and metastasis of ATC in vivo. Notably, knockdown of NUF2 epigenetically inhibited the expression of magnesium transporters through reducing the abundance of H3K4me3 at promoters, thereby reduced intracellular Mg2+ concentration. Furthermore, we found the deletion of NUF2 or magnesium transporters significantly inhibited the protein synthesis mediated by the PI3K/Akt/mTOR pathway. In conclusion, NUF2 functions as an emerging regulator for protein synthesis by maintaining the homeostasis of intracellular Mg2+, which finally drives ATC progression.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
MeSH terms
Substances
Grants and funding
- U20A20382/National Natural Science Foundation of China (National Science Foundation of China)
- 82273287/National Natural Science Foundation of China (National Science Foundation of China)
- 82161138019/National Natural Science Foundation of China (National Science Foundation of China)
- 82173157/National Natural Science Foundation of China (National Science Foundation of China)
- LY22H160036/Natural Science Foundation of Zhejiang Province (Zhejiang Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

