Cell swelling enhances ligand-driven β-adrenergic signaling
- PMID: 39242606
- PMCID: PMC11379887
- DOI: 10.1038/s41467-024-52191-y
Cell swelling enhances ligand-driven β-adrenergic signaling
Abstract
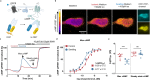
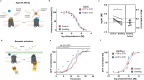
G protein-coupled receptors' conformational landscape can be affected by their local, microscopic interactions within the cell plasma membrane. We employ here a pleiotropic stimulus, namely osmotic swelling, to alter the cortical environment within intact cells and monitor the response in terms of receptor function and downstream signaling. We observe that in osmotically swollen cells the β2-adrenergic receptor, a prototypical GPCR, favors an active conformation, resulting in cAMP transient responses to adrenergic stimulation that have increased amplitude. The results are validated in primary cell types such as adult cardiomyocytes, a model system where swelling occurs upon ischemia-reperfusion injury. Our results suggest that receptors' function is finely modulated by their biophysical context, and specifically that osmotic swelling acts as a potentiator of downstream signaling, not only for the β2-adrenergic receptor, but also for other receptors, hinting at a more general regulatory mechanism.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

